Cleavable Linker Incorporation into a Synthetic Dye-Nanobody-Fluorescent Protein Assembly: FRET, FLIM and STED Microscopy
- PMID: 35838445
- PMCID: PMC9804610
- DOI: 10.1002/cbic.202200395
Cleavable Linker Incorporation into a Synthetic Dye-Nanobody-Fluorescent Protein Assembly: FRET, FLIM and STED Microscopy
Abstract
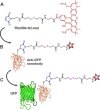
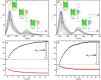
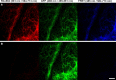
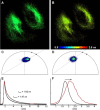
A bright and photostable fluorescent dye with a disulfide (S-S) linker and maleimide group (Rho594-S2-mal), as cleavable and reactive sites, was synthesized and conjugated with anti-GFP nanobodies (NB). The binding of EGFP (FRET donor) with anti-GFP NB labeled with one or two Rho594-S2-mal residues was studied in vitro and in cellulo. The linker was cleaved with dithiothreitol recovering the donor (FP) signal. The bioconjugates (FP-NB-dye) were applied in FRET-FLIM assays, confocal imaging, and superresolution STED microscopy.
Keywords: FRET; STED microscopy; dyes / pigments; fluorescence; nanobodies.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In Vivo Interaction Studies by Measuring Förster Resonance Energy Transfer Through Fluorescence Lifetime Imaging Microscopy (FRET/FLIM).Methods Mol Biol. 2017;1662:159-170. doi: 10.1007/978-1-4939-7262-3_14. Methods Mol Biol. 2017. PMID: 28861826
-
Quantitative comparison of different fluorescent protein couples for fast FRET-FLIM acquisition.Biophys J. 2009 Oct 21;97(8):2368-76. doi: 10.1016/j.bpj.2009.07.044. Biophys J. 2009. PMID: 19843469 Free PMC article.
-
Visualization of Bacterial Protein Complexes Labeled with Fluorescent Proteins and Nanobody Binders for STED Microscopy.Int J Mol Sci. 2019 Jul 10;20(14):3376. doi: 10.3390/ijms20143376. Int J Mol Sci. 2019. PMID: 31295803 Free PMC article.
-
Optical methods in the study of protein-protein interactions.Adv Exp Med Biol. 2010;674:33-42. doi: 10.1007/978-1-4419-6066-5_4. Adv Exp Med Biol. 2010. PMID: 20549938 Review.
-
Monitoring protein interactions in living cells with fluorescence lifetime imaging microscopy.Methods Enzymol. 2012;504:371-91. doi: 10.1016/B978-0-12-391857-4.00019-7. Methods Enzymol. 2012. PMID: 22264545 Free PMC article. Review.
Cited by
-
Multicolor lifetime imaging and its application to HIV-1 uptake.Nat Commun. 2023 Aug 17;14(1):4994. doi: 10.1038/s41467-023-40731-x. Nat Commun. 2023. PMID: 37591879 Free PMC article.
-
Measuring Metabolic Changes in Cancer Cells Using Two-Photon Fluorescence Lifetime Imaging Microscopy and Machine-Learning Analysis.J Biophotonics. 2025 Jan;18(1):e202400426. doi: 10.1002/jbio.202400426. Epub 2024 Nov 25. J Biophotonics. 2025. PMID: 39587841 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

