Compression-coated pulsatile chronomodulated therapeutic system: QbD assisted optimization
- PMID: 35838522
- PMCID: PMC9477481
- DOI: 10.1080/10717544.2022.2094500
Compression-coated pulsatile chronomodulated therapeutic system: QbD assisted optimization
Abstract
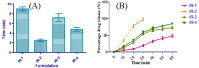
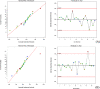
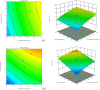
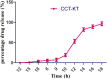
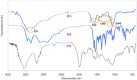
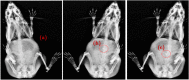
Pulsatile drug delivery systems have drawn attention in contemporary research for designing chronotherapeutic systems. The current work aims to design pulsatile ketorolac tromethamine tablets using compression coating for delayed delivery with a lag time suitable for the treatment of morning stiffness in arthritis. Rapidly disintegrating core tablets of ketorolac tromethamine were formulated using super-disintegrants, and the optimized formulation was compression using PEO WSR coagulant and Eudragit RLPO for delaying the release. The central composite design and response surface methodology were employed to optimize the formulation and process parameters namely PEO WSR Coagulant (X1), Eudragit RLPO (X2), and Hardness (X3). The dependent variables optimized were lag time and time required for 95% drug release. Analysis using response surface graphs and mathematical modeling of the results allowed identifying and quantifying the formulation variables active on the selected responses. A polynomial equation fitted to the data was used to predict the composition with optimum responses. Compression-coated pulsatile tablets' optimized composition exhibited a lag time of 9 h and released 95% of the ketorolac tromethamine in 17.42 h. Validation of the mathematical model assured the reliability of QBD in formulation design. In vivo X-ray imaging and pharmacokinetic studies established a strong relationship between the coated polymers maintaining the desired lag time for delayed delivery of the active to coincide with the chronobiology for enhanced bioavailability at the right time when needed.
Keywords: Compression coating; Eudragit RLPO; PEO WSR coagulant; chronobiology; ketorolac tromethamine; quality by design.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


Similar articles
-
Formulation and pharmacokinetics of colon-specific double-compression coated mini-tablets: Chronopharmaceutical delivery of ketorolac tromethamine.Int J Pharm. 2015 Aug 1;491(1-2):35-41. doi: 10.1016/j.ijpharm.2015.06.007. Epub 2015 Jun 6. Int J Pharm. 2015. PMID: 26056929
-
Development, evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets.Expert Opin Drug Deliv. 2013 Jan;10(1):33-45. doi: 10.1517/17425247.2013.743528. Epub 2012 Nov 30. Expert Opin Drug Deliv. 2013. PMID: 23199134
-
Iron oxide induced enhancement of mucoadhesive potential of Eudragit RLPO: formulation, evaluation and optimization of mucoadhesive drug delivery system.Expert Opin Drug Deliv. 2013 Sep;10(9):1179-91. doi: 10.1517/17425247.2013.790361. Epub 2013 Apr 17. Expert Opin Drug Deliv. 2013. PMID: 23590289
-
Mini-tablets: a contemporary system for oral drug delivery in targeted patient groups.Expert Opin Drug Deliv. 2015 Jan;12(1):65-84. doi: 10.1517/17425247.2014.951633. Epub 2014 Sep 9. Expert Opin Drug Deliv. 2015. PMID: 25200881 Review.
-
Oral pulsatile delivery: rationale and chronopharmaceutical formulations.Int J Pharm. 2010 Oct 15;398(1-2):1-8. doi: 10.1016/j.ijpharm.2010.07.026. Epub 2010 Jul 23. Int J Pharm. 2010. PMID: 20655998 Review.
Cited by
-
Optimization of process parameters for fabrication of electrospun nanofibers containing neomycin sulfate and Malva sylvestris extract for a better diabetic wound healing.Drug Deliv. 2022 Dec;29(1):3370-3383. doi: 10.1080/10717544.2022.2144963. Drug Deliv. 2022. PMID: 36404771 Free PMC article.
-
Enhancement of antifungal activity and transdermal delivery of 5-flucytosine via tailored spanlastic nanovesicles: statistical optimization, in-vitro characterization, and in-vivo biodistribution study.Front Pharmacol. 2023 Dec 4;14:1321517. doi: 10.3389/fphar.2023.1321517. eCollection 2023. Front Pharmacol. 2023. PMID: 38125883 Free PMC article.
-
Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study.Molecules. 2022 Nov 16;27(22):7914. doi: 10.3390/molecules27227914. Molecules. 2022. PMID: 36432014 Free PMC article.
-
QbD driven targeted pulmonary delivery of dexamethasone-loaded chitosan microspheres: Biodistribution and pharmacokinetic study.Saudi Pharm J. 2023 Sep;31(9):101711. doi: 10.1016/j.jsps.2023.101711. Epub 2023 Jul 26. Saudi Pharm J. 2023. PMID: 37564747 Free PMC article.
-
Design and Evaluation of S-Protected Thiolated-Based Itopride Hydrochloride Polymeric Nanocrystals for Functional Dyspepsia: QbD-Driven Optimization, In Situ, In Vitro, and In Vivo Investigation.Pharmaceuticals (Basel). 2023 Jun 25;16(7):925. doi: 10.3390/ph16070925. Pharmaceuticals (Basel). 2023. PMID: 37513837 Free PMC article.
References
-
- Abdellatif AAH, Mohammed AM, Zayed G, et al. (2022). Cyclodextrin/adamantane-grafted polyethylene glycol-based self-assembling constructs for topical delivery of ketorolac tromethamine: formulation, characterization, and in vivo studies. AAPS PharmSciTech 23:45. - PubMed
-
- Adetunji AI, Olaniran AO. (2020). Statistical modelling and optimization of protease production by an autochthonous Bacillus aryabhattai Ab15-ES: a response surface methodology approach. Biocatal Agric Biotechnol 24:101528.
-
- Ahmed OAA, Kurakula M, Banjar ZM, et al. (2015). Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J Pharm Sci 104:2062–75. - PubMed
-
- BaHammam A, Alrajeh M, Albabtain M, et al. (2010). Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite 54:426–9. - PubMed
-
- Buttgereit F, Smolen JS, Coogan AN, Cajochen C. (2015). Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol 11:349–56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
