The current role and future directions of imaging in failed back surgery syndrome patients: an educational review
- PMID: 35838802
- PMCID: PMC9287525
- DOI: 10.1186/s13244-022-01246-z
The current role and future directions of imaging in failed back surgery syndrome patients: an educational review
Abstract
Background: Failed back surgery syndrome (FBSS) is an umbrella term referring to painful sensations experienced by patients after spinal surgery, mostly of neuropathic nature. Adequate treatment of FBSS is challenging, as its etiology is believed to be multifactorial and still not fully clarified. Accurate identification of the source of pain is difficult but pivotal to establish the most appropriate treatment strategy. Although the clinical utility of imaging in FBSS patients is still contentious, objective parameters are highly warranted to map different phenotypes of FBSS and tailor each subsequent therapy.
Main body: Since technological developments have weakened the applicability of prior research, this educational review outlined the recent evidence (i.e., from January 2005 onwards) after a systematic literature search. The state of the art on multiple imaging modalities in FBSS patients was reviewed. Future directions related to functional MRI and the development of imaging biomarkers have also been discussed.
Conclusion: Besides the fact that more imaging studies correlated with symptomatology in the postoperative setting are warranted, the current educational review outlined that contrast-enhanced MRI and MR neurography have been suggested as valuable imaging protocols to assess alterations in the spine of FBSS patients. The use of imaging biomarkers to study correlations between imaging features and symptomatology might hold future potential; however, more research is required before any promising hypotheses can be drawn.
Keywords: Artificial intelligence; Diagnostic imaging; Failed back surgery syndrome; Magnetic resonance imaging; Signs and symptoms.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

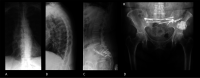
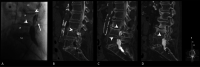
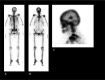
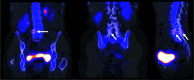
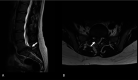
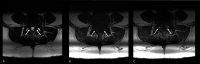
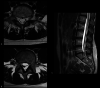

Similar articles
-
Increasing Rates of Imaging in Failed Back Surgery Syndrome Patients: Implications for Spinal Cord Stimulation.Pain Physician. 2017 Sep;20(6):E969-E977. Pain Physician. 2017. PMID: 28934801 Free PMC article.
-
Failed back surgery syndrome: what's in a name? A proposal to replace "FBSS" by "POPS"….Neurochirurgie. 2015 Mar;61 Suppl 1:S16-21. doi: 10.1016/j.neuchi.2014.12.001. Epub 2015 Feb 7. Neurochirurgie. 2015. PMID: 25665773
-
Etiology, Evaluation, and Treatment of Failed Back Surgery Syndrome.Asian Spine J. 2018 Jun;12(3):574-585. doi: 10.4184/asj.2018.12.3.574. Epub 2018 Jun 4. Asian Spine J. 2018. PMID: 29879788 Free PMC article. Review.
-
Salience, central executive, and sensorimotor network functional connectivity alterations in failed back surgery syndrome.Scand J Pain. 2017 Jul;16:10-14. doi: 10.1016/j.sjpain.2017.01.008. Epub 2017 Feb 20. Scand J Pain. 2017. PMID: 28850382
-
Treatment Options for Failed Back Surgery Syndrome Patients With Refractory Chronic Pain: An Evidence Based Approach.Spine (Phila Pa 1976). 2017 Jul 15;42 Suppl 14:S41-S52. doi: 10.1097/BRS.0000000000002217. Spine (Phila Pa 1976). 2017. PMID: 28505029 Review.
Cited by
-
Molecular imaging techniques in patients with persistent spinal pain syndrome type 2 - a systematic review and meta-analysis.EJNMMI Rep. 2025 Aug 25;9(1):30. doi: 10.1186/s41824-025-00266-4. EJNMMI Rep. 2025. PMID: 40851058 Free PMC article. Review.
-
High-Frequency Bipolar Coagulation Limits Epidural Fibrosis in Lumbar Microdiscectomy.Cureus. 2023 Sep 12;15(9):e45077. doi: 10.7759/cureus.45077. eCollection 2023 Sep. Cureus. 2023. PMID: 37705564 Free PMC article.
-
Topical administration of tranexamic acid for prevention of postoperative epidural fibrosis: insights from a rabbit laminectomy model.Vet Res Forum. 2025;16(3):181-187. doi: 10.30466/vrf.2024.2031220.4312. Epub 2025 Mar 15. Vet Res Forum. 2025. PMID: 40391134 Free PMC article.
-
[18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis.Brain Sci. 2025 Jul 7;15(7):724. doi: 10.3390/brainsci15070724. Brain Sci. 2025. PMID: 40722315 Free PMC article.
-
Failed back surgery syndrome-terminology, etiology, prevention, evaluation, and management: a narrative review.J Yeungnam Med Sci. 2024 Jul;41(3):166-178. doi: 10.12701/jyms.2024.00339. Epub 2024 Jun 10. J Yeungnam Med Sci. 2024. PMID: 38853538 Free PMC article.
References
-
- Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2. IASP Press; 1994. p. 222.
LinkOut - more resources
Full Text Sources

