Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer's dementia
- PMID: 35838824
- PMCID: PMC9381462
- DOI: 10.1007/s00401-022-02467-8
Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer's dementia
Abstract
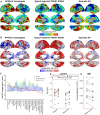
We describe in vivo follow-up PET imaging and postmortem findings from an autosomal dominant Alzheimer's disease (ADAD) PSEN1 E280A carrier who was also homozygous for the APOE3 Christchurch (APOE3ch) variant and was protected against Alzheimer's symptoms for almost three decades beyond the expected age of onset. We identified a distinct anatomical pattern of tau pathology with atypical accumulation in vivo and unusual postmortem regional distribution characterized by sparing in the frontal cortex and severe pathology in the occipital cortex. The frontal cortex and the hippocampus, less affected than the occipital cortex by tau pathology, contained Related Orphan Receptor B (RORB) positive neurons, homeostatic astrocytes and higher APOE expression. The occipital cortex, the only cortical region showing cerebral amyloid angiopathy (CAA), exhibited a distinctive chronic inflammatory microglial profile and lower APOE expression. Thus, the Christchurch variant may impact the distribution of tau pathology, modulate age at onset, severity, progression, and clinical presentation of ADAD, suggesting possible therapeutic strategies.
Keywords: APOE; Alzheimer’s disease; Dementia; PET; Tau; Transcriptomics.
© 2022. The Author(s).
Conflict of interest statement
RAS has received personal compensation from AC Immune, Eisai, Roche, and Takeda, and research grant support from Eli Lilly and Janssen. YTQ, JFAV, EMR, FL are listed as co-inventors of a patent application for therapeutics modulating interactions between APOE and HSPG filed by MGB. All other authors have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

