Bioabsorbable implants in forefoot surgery: a review of materials, possibilities and disadvantages
- PMID: 35839087
- PMCID: PMC8693227
- DOI: 10.1302/2058-5241.6.200157
Bioabsorbable implants in forefoot surgery: a review of materials, possibilities and disadvantages
Abstract
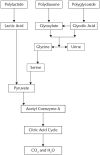
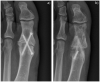
Bioabsorbable and biodegradable implants offer new possibilities in orthopaedic and trauma surgery. As soon as the initial stability of the degradable implants has reached the qualities of conventional materials, new devices may find usage in younger and more demanding patients. Residual conventional osteosynthetic material or the necessity to remove metal increasingly seems to be more of an adverse event than daily practice in forefoot surgery. Nevertheless, some drawbacks need to be discussed. Recent literature screened for the use of bioabsorbable and biodegradable materials in forefoot surgery, available implants and indications in forefoot surgery were analysed and summarized. Apart from common indications in forefoot surgery, points of interest were the type of biomaterial, the process of biodegradation and biointegration, and possible adverse events. Materials were comprehensively discussed for each indication based on the available literature. Polylactide, polyglycoside and polydioxanone are considered safe and sufficiently stable for use in forefoot surgery. Low complication rates (e.g. 0.7% for pin fixation in hallux deformities) are given. Magnesium implants suffered from an extensive corrosive process in the first generation but now seem to be safe in forefoot surgery and offer good options compared with conventional titanium screws, especially in procedures of the first ray. Allograft bone has proven feasibility in small case series, but still lacks larger or randomized clinical trials. The first results are promising. Bioresorbable and osseointegrating devices offer attractive new possibilities for surgeons and patients. Despite all the known advantages, the difficulties and possible complications must not be forgotten, such as soft tissue reactions, unwanted osteolysis and a lower primary mechanical load capacity.
Conflict of interest statement
Figures
References
-
- Mäkelä EA, Böstman O, Kekomäki M, et al. . Biodegradable fixation of distal humeral physeal fractures. Clin Orthop Relat Res 1992;283:237–243. - PubMed
-
- Juutilainen T, Pätiälä H, Rokkanen P, Törmälä P. Biodegradable wire fixation in olecranon and patella fractures combined with biodegradable screws or plugs and compared with metallic fixation. Arch Orthop Trauma Surg 1995;114:319–323. - PubMed
-
- Stroud CC. Absorbable implants in fracture management. Foot Ankle Clin 2002;7:495–499. - PubMed
-
- Illi OE, Weigum H, Misteli F. Biodegradable implant materials in fracture fixation. Clin Mater 1992;10:69–73. - PubMed
-
- Kulkarni RK, Moore EG, Hegyeli AF, Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res 1971;5:169–181. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials