Acidic pH Promotes Refolding and Macroscopic Assembly of Amyloid β (16-22) Peptides at the Air-Water Interface
- PMID: 35839425
- PMCID: PMC9340808
- DOI: 10.1021/acs.jpclett.2c01171
Acidic pH Promotes Refolding and Macroscopic Assembly of Amyloid β (16-22) Peptides at the Air-Water Interface
Abstract
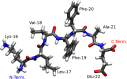
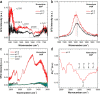
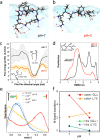
Assembly by amyloid-beta (Aβ) peptides is vital for various neurodegenerative diseases. The process can be accelerated by hydrophobic interfaces such as the cell membrane interface and the air-water interface. Elucidating the assembly mechanism for Aβ peptides at hydrophobic interface requires knowledge of the microscopic structure of interfacial peptides. Here we combine scanning force microscopy, sum-frequency generation spectroscopy, and metadynamics simulations to probe the structure of the central fragment of Aβ peptides at the air-water interface. We find that the structure of interfacial peptides depends on pH: at neutral pH, the peptides adopt a less folded, bending motif by forming intra-hydrogen bonds; at acidic pH, the peptides refold into extended β-strand fibril conformation, which further promotes their macroscopic assembly. The conformational transition of interfacial peptides is driven by the reduced hydrogen bonds, both with water and within peptides, resulting from the protonation of acidic glutamic acid side chains.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Role of water in protein aggregation and amyloid polymorphism.Acc Chem Res. 2012 Jan 17;45(1):83-92. doi: 10.1021/ar2000869. Epub 2011 Jul 15. Acc Chem Res. 2012. PMID: 21761818 Free PMC article.
-
Computational Study on the Assembly of Amyloid β-Peptides in the Hydrophobic Environment.Chem Pharm Bull (Tokyo). 2019;67(9):959-965. doi: 10.1248/cpb.c19-00171. Chem Pharm Bull (Tokyo). 2019. PMID: 31474736
-
Effect of the air-water interface on the conformation of amyloid beta.Biointerphases. 2020 Dec 17;15(6):061011. doi: 10.1116/6.0000620. Biointerphases. 2020. PMID: 33334114 Free PMC article.
-
Molecular Dynamics Simulation Studies on the Aggregation of Amyloid-β Peptides and Their Disaggregation by Ultrasonic Wave and Infrared Laser Irradiation.Molecules. 2022 Apr 12;27(8):2483. doi: 10.3390/molecules27082483. Molecules. 2022. PMID: 35458686 Free PMC article. Review.
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
Cited by
-
True Origin of Amide I Shifts Observed in Protein Spectra Obtained with Sum Frequency Generation Spectroscopy.J Phys Chem Lett. 2023 Jun 1;14(21):4949-4954. doi: 10.1021/acs.jpclett.3c00391. Epub 2023 May 22. J Phys Chem Lett. 2023. PMID: 37213084 Free PMC article.
-
Multiscale Simulations of Self-Assembling Peptides: Surface and Core Hydrophobicity Determine Fibril Stability and Amyloid Aggregation.Biomacromolecules. 2024 May 13;25(5):3063-3075. doi: 10.1021/acs.biomac.4c00151. Epub 2024 Apr 23. Biomacromolecules. 2024. PMID: 38652055 Free PMC article.
-
Fibril formation and ordering of disordered FUS LC driven by hydrophobic interactions.Nat Chem. 2023 Aug;15(8):1146-1154. doi: 10.1038/s41557-023-01221-1. Epub 2023 May 25. Nat Chem. 2023. PMID: 37231298 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

