Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma
- PMID: 35839452
- PMCID: PMC10653042
- DOI: 10.1182/blood.2022015478
Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma
Abstract
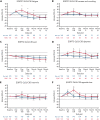
Here, we report the first comparative analysis of patient-reported outcomes (PROs) with chimeric antigen receptor T-cell therapy vs standard-of-care (SOC) therapy in second-line relapsed/refractory large B-cell lymphoma (R/R LBCL) from the pivotal randomized phase 3 ZUMA-7 study of axicabtagene ciloleucel (axi-cel) vs SOC. PRO instruments were administered at baseline, day 50, day 100, day 150, month 9, and every 3 months from randomization until 24 months or an event-free survival event. The quality of life (QoL) analysis set comprised patients with a baseline and ≥1 follow-up PRO completion. Prespecified hypotheses for Quality of Life Questionnaire-Core 30 (QLQ-C30) physical functioning, global health status/QoL, and EQ-5D-5L visual analog scale (VAS) were tested using mixed-effects models with repeated measures. Clinically meaningful changes were defined as 10 points for QLQ-C30 and 7 for EQ-5D-5L VAS. Among 359 patients, 296 (165 axi-cel, 131 SOC) met inclusion criteria for QoL analysis. At day 100, statistically significant and clinically meaningful differences in mean change of scores from baseline were observed favoring axi-cel over SOC for QLQ-C30 global health status/QoL (estimated difference 18.1 [95% confidence interval (CI), 12.3-23.9]), physical functioning (13.1 [95% CI, 8.0-18.2]), and EQ-5D-5L VAS (13.7 [95% CI, 8.5-18.8]; P < .0001 for all). At day 150, scores significantly favored axi-cel vs SOC for global health status/QoL (9.8 [95% CI, 2.6-17.0]; P = .0124) and EQ-5D-5L VAS (11.3 [95% CI, 5.4-17.1]; P = .0004). Axi-cel showed clinically meaningful improvements in QoL over SOC. Superior clinical outcomes and favorable patient experience with axi-cel should help inform treatment choices in second-line R/R LBCL. This trial was registered at www.clinicaltrials.gov as #NCT03391466.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.E. reports honoraria from Kite, Bristol Myers Squibb, Novartis, Pfizer, and Janssen and consulting or advisory role with Kite, Bristol Myers Squibb, Novartis, Pfizer, and Janssen. J.C.C. reports consultancy fees for Kite, AbbVie, Janssen, ADC Therapeutics, Karyopharm, Kymera, Genmab, and Novartis; speakers' bureau participation for Morphosys, Epizyme, Bristol Myers Squibb, BeiGene, and AstraZeneca; and research funding from AstraZeneca, Merck, ADC Therapeutics, and Adaptive. I.A. reports speakers’ bureau participation for Kite and Novartis. L.W. reports consultancy or advisory role for Novartis, MSD, Bristol Myers Squibb, AstraZeneca, and Roche; research funding from Roche; and expert testimony from AstraZeneca and Novartis. K.C. reports consultancy or advisory role for Kite, Roche, Takeda, Celgene, Atara, Gilead, Janssen, and Incyte; speakers' bureau for Roche, Takeda, Kite, Gilead, and Incyte; and travel support from Roche, Takeda, Kite, Janssen, and Bristol Myers Squibb/Celgene. K.O. reports consultancy or advisory role for Kite. K. Davison reports consulting or advisory role for Merck, AstraZeneca, Janssen, Novartis, Gilead, AbbVie, and Celgene. J.D.R. reports consultancy or advisory role with Kite, Novartis, Amgen, MSD, Roche, and Bristol Myers Squibb/Celgene; speakers' bureau for Kite, Novartis, and Roche; and research funding from Kite and Novartis. S.D. reports consultancy or advisory role for Kite, Atara Biotherapeutics, and Bristol Myers Squibb and research funding from Jazz Pharmaceuticals and Miltenyi Biotech. K. Dorritie reports research funding from Kite, Juno/Bristol Myers Squibb, Hoffman-LaRoche, Janssen, and Genmab. S.J. reports consultancy or advisory role for Kite, Juno, Novartis, Takeda, and CRISPR Therapeutics and research funding from Kite and Novartis. J.R. reports stock or other ownership in ADC Therapeutics and AstraZeneca; honoraria from Takeda, ADCT, and Bristol Myers Squibb; consultancy or advisory role for Takeda, ADCT, Bristol Myers Squibb, and Novartis; speakers' bureau from Takeda and ADCT; research funding from Takeda; and expert testimony from Takeda and ADCT. F.M. reports consultancy or advisory role for Roche, Bristol Myers Squibb, Gilead, Epizyme, Genmab, Novartis, and AbbVie; speakers’ bureau from Roche; and expert testimony from Roche and Genentech. D.C. reports research funding from MedImmune, AstraZeneca, Clovis, Eli Lilly, 4SC, Bayer, Celgene, and Roche. A.M.G.-S. reports honoraria from Roche, Celgene, Janssen, Servier, and Gilead; consulting or advisory role with Roche, Bristol Myers Squibb/Celgene, Morphosys, Kyowa Kirin, Clinigen, Eusa Pharma, Novartis, Gilead, Servier, and Incyte; research funding from Janssen; expert testimony from Gilead; and travel support Roche, Celgene, Servier, and Kern Pharma. D.T. reports consultancy or advisory role for Partner, Takeda, EUSA, Kite, Kyowa Kirin, and Magenta; speakers' bureau participation for Takeda and Kite; and research funding from Bristol Myers Squibb, Kite, Genentech, Incyte, and Fate Therapeutics. R.K. reports consulting or advisory role with BeiGene, Kite, Morphosys, Karyopharm, Bristol Myers Squibb/Celgene, Genentech, Roche, Janssen, and Pharmacyclics; speakers’ bureau from AstraZeneca, BeiGene, Kite, and Morphosys; and research funding from Kite, Bristol Myers Squibb/Celgene, and Takeda. N.K. reports honoraria from Gilead, Novartis, and Celgene and consultancy or advisory role for Gilead, Novartis, and Celgene. C. Thieblemont reports honoraria from Bristol Myers Squibb/Celgene, AbbVie, Takeda, Novartis, Roche, Kite and Incyte; consultancy or advisory role for Bristol Myers Squibb/Celgene, AbbVie, Takeda, Roche, Novartis, Kite, and Incyte; and travel support from Bristol Myers Squibb/Celgene, Takeda, Roche, Novartis, and Kite. G.E. reports consultancy or advisory role with Kite and Gilead Sciences. P.D. reports consulting or advisory role for AbbVie, AstraZeneca, bluebird bio, Bristol Myers Squibb, Gilead Sciences, Janssen, and Novartis and research funding from Riemser. R.M. reports honoraria from Gilead; consultancy or advisory role Gilead Science; and travel support from Gilead. N.J., W.-J.W., and C.T.S. report employment with OPEN Health; consultancy or advisory role for Kite through employment at OPEN Health; and research funding from multiple clients through employment at OPEN Health. J.T.S. and C. To report employment with Kite and stock or other ownership in Gilead. P.C. reports employment with Kite; stock or other ownership in Gilead; and travel support from Kite. M.J.K. reports honoraria from Kite, Novartis, Miltenyi Biotech, Roche, and Bristol Myers Squibb/Celgene; consultancy or advisory role for Kite, Roche, Bristol Myers Squibb/Celgene, Novartis, and Miltenyi Biotech; research funding from Kite, Roche, Takeda, and Celgene; and travel support from Kite, Roche, Novartis, and Miltenyi Biotech. The remaining authors declare no competing financial interests.
Figures





Comment in
-
Axi-cel in LBCL: fulfill two needs with one deed.Blood. 2022 Nov 24;140(21):2183-2185. doi: 10.1182/blood.2022017564. Blood. 2022. PMID: 36422859 No abstract available.
References
-
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. - PubMed
-
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

