"Ventilator-free days" composite outcome in patients with SARS-CoV-2 infection treated with tocilizumab: A retrospective competing risk analysis
- PMID: 35839546
- PMCID: PMC9242887
- DOI: 10.1016/j.hrtlng.2022.06.024
"Ventilator-free days" composite outcome in patients with SARS-CoV-2 infection treated with tocilizumab: A retrospective competing risk analysis
Abstract
Background: SARS-CoV-2 infection demonstrates a wide range of severity, with more severe cases presenting with a cytokine storm with elevated serum interleukin-6; hence, the interleukin-6 receptor antibody tocilizumab was used for the management of severe cases.
Objective: To explore the effect of tocilizumab on ventilator-free day composite outcomes among critically ill patients with SARS-CoV-2 infection.
Methods: This retrospective propensity score-matching study compared mechanically ventilated patients who received tocilizumab to a control group.
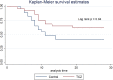
Results: Twenty-nine patients in the intervention group were compared to 29 controls. The matched groups were similar. The ventilator-free days composite outcome was higher in the intervention group (sub-distribution hazard ratio 2.7, 95% confidence interval [CI]: 1.2-6.3; p = 0.02), the mortality rate in the intensive care unit was not different (37.9% vs 62%, p = 0.1), and actual ventilator-free days were significantly longer in the tocilizumab group (mean difference 4.7 days; p = 0.02). Sensitivity analysis showed a significantly lower hazard ratio for death in the tocilizumab group (HR 0.49, 95% CI: 0.25-0.97; p = 0.04). Positive cultures were not significantly different among the groups (55.2% vs 34.5% in the tocilizumab and control groups, respectively; p = 0.1).
Conclusions: Tocilizumab may improve the composite outcome of ventilator-free days at day 28 among mechanically ventilated patients with SARS-CoV-2 infection. It is associated with significantly longer actual ventilator-free days, insignificantly lower mortality, and higher superinfection.
Keywords: Mechanical ventilation; SARS-CoV-2; Tocilizumab; Ventilator-free days.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Nothing to declare.
Figures
References
-
- World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [accessed 13 March 2022 ].
-
- Aletreby W.T., Alharthy A.M., Faqihi F., et al. Dynamics of SARS CoV-2 outbreak in the Kingdom of Saudi Arabia: a predictive model. Saudi Crit Care J. 2020;4:79–83. doi: 10.4103/sccj.sccj_19_20. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous