Mucosal chemokine adjuvant enhances synDNA vaccine-mediated responses to SARS-CoV-2 and provides heterologous protection in vivo
- PMID: 35839767
- PMCID: PMC9237025
- DOI: 10.1016/j.xcrm.2022.100693
Mucosal chemokine adjuvant enhances synDNA vaccine-mediated responses to SARS-CoV-2 and provides heterologous protection in vivo
Abstract
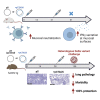
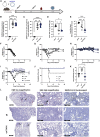
The global coronavirus disease 2019 (COVID-19) pandemic has claimed more than 5 million lives. Emerging variants of concern (VOCs) continually challenge viral control. Directing vaccine-induced humoral and cell-mediated responses to mucosal surfaces may enhance vaccine efficacy. Here we investigate the immunogenicity and protective efficacy of optimized synthetic DNA plasmids encoding wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (pS) co-formulated with the plasmid-encoded mucosal chemokine cutaneous T cell-attracting chemokine (pCTACK; CCL27). pCTACK-co-immunized animals exhibit increased spike-specific antibodies at the mucosal surface and increased frequencies of interferon gamma (IFNγ)+ CD8+ T cells in the respiratory mucosa. pCTACK co-immunization confers 100% protection from heterologous Delta VOC challenge. This study shows that mucosal chemokine adjuvants can direct vaccine-induced responses to specific immunological sites and have significant effects on heterologous challenge. Further study of this unique chemokine-adjuvanted vaccine approach in the context of SARS-CoV-2 vaccines is likely important.
Keywords: COVID-19; DNA vaccine; SARS-CoV-2; adjuvant; chemokine; mucosal.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interests D.B.W. has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees and board series. Remuneration received by D.B.W. includes direct payments and stock or stock options. D.B.W. discloses the following paid associations with commercial partners: GeneOne (consultant), Geneos (advisory board), AstraZeneca (advisory board, speaker), Inovio (BOD, SRA, stock), Sanofi (advisory board), and BBI (advisory board).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

