Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis
- PMID: 35839811
- PMCID: PMC9296658
- DOI: 10.1016/S2214-109X(22)00241-8
Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis
Erratum in
-
Correction to Lancet Glob Health 2022; 10: e1115-27.Lancet Glob Health. 2023 Jul;11(7):e1011. doi: 10.1016/S2214-109X(23)00240-1. Epub 2023 May 15. Lancet Glob Health. 2023. PMID: 37201544 Free PMC article. No abstract available.
Abstract
Background: Cervical cancer screening coverage is a key monitoring indicator of the WHO cervical cancer elimination plan. We present global, regional, and national cervical screening coverage estimates against the backdrop of the 70% coverage target set by WHO.
Methods: In this review and synthetic analysis, we searched scientific literature, government websites, and official documentation to identify official national recommendations and coverage data for cervical cancer screening for the 194 WHO member states and eight associated countries and territories published from database inception until Oct 30, 2020, supplemented with a formal WHO country consultation from Nov 27, 2020, to Feb 12, 2021. We extracted data on the year of introduction of recommendations, the existence of individual invitation to participate, financing of screening tests, primary screening and triage tests used, recommended ages and screening intervals, use of self-sampling, and use of screen-and-treat approaches. We also collected coverage data, either administrative or survey-based, as disaggregated as possible by age and for any available screening interval. According to data completeness and representativeness, different statistical models were developed to produce national age-specific coverages by screening interval, which were transformed into single-age datapoints. Missing data were imputed. Estimates were applied to the 2019 population and aggregated by region and income level.
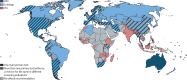
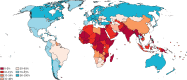
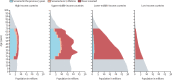
Findings: We identified recommendations for cervical screening in 139 (69%) of 202 countries and territories. Cytology was the primary screening test in 109 (78%) of 139 countries. 48 (35%) of 139 countries recommended primary HPV-based screening. Visual inspection with acetic acid was the most recommended test in resource-limited settings. Estimated worldwide coverage in women aged 30-49 years in 2019 was 15% in the previous year, 28% in the previous 3 years, and 32% in the previous 5 years, and 36% ever in lifetime. An estimated 1·6 billion (67%) of 2·3 billion women aged 20-70 years, including 662 million (64%) of 1·0 billion women aged 30-49 years, had never been screened for cervical cancer. 133 million (84%) of 158 million women aged 30-49 years living in high-income countries had been screened ever in lifetime, compared with 194 million (48%) of 404 million women in upper-middle-income countries, 34 million (9%) of 397 million women in lower-middle-income countries, and 8 million (11%) of 74 million in low-income countries.
Interpretation: Two in three women aged 30-49 years have never been screened for cervical cancer. Roll-out of screening is very low in low-income and middle-income countries, where the burden of disease is highest. The priority of the WHO elimination campaign should be to increase both screening coverage and treatment of detected lesions; however, expanding the efforts of surveillance systems in both coverage and quality control are major challenges to achieving the WHO elimination target.
Funding: Instituto de Salud Carlos III, European Regional Development Fund, Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia, and Horizon 2020.
Translations: For the French, Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2022 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY NC ND 3.0 IGO license which permits users to download and share the article for non-commercial purposes, so long as the article is reproduced in the whole without changes, and provided the original source is properly cited. This article shall not be used or reproduced in association with the promotion of commercial products, services or any entity. There should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests The Cancer Epidemiology Research Programme (with which LB, BS, ER, and LA are affiliated) has received sponsorship for grants from Merck Sharp & Dohme and HPV test kits at no cost from Roche for research purposes. All other authors report no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of WHO. Authors who are identified as personnel of the International Agency for Research on Cancer, WHO, are responsible alone for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer, WHO.
Figures
References
-
- Vaccarella S, Lortet-Tieulent J, Saracci R, Conway DI, Straif K, Wild CP. International Agency for Research on Cancer; Lyon, France: 2019. Reducing social inequalities in cancer: evidence and priorities for research. - PubMed
-
- WHO Global strategy to accelerate the elimination of cervical cancer as a public health problem. Nov 17, 2020. https://www.who.int/publications-detail-redirect/9789240014107
-
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. - PubMed
-
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88–F99. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

