Safety and effectiveness of the fluocinolone acetonide intravitreal implant (ILUVIEN): 3-year results from the European IRISS registry study
- PMID: 35840291
- PMCID: PMC10579189
- DOI: 10.1136/bjo-2022-321415
Safety and effectiveness of the fluocinolone acetonide intravitreal implant (ILUVIEN): 3-year results from the European IRISS registry study
Abstract
Background: The ILUVIEN Registry Safety Study was a multicentre, open-label, non-randomised, observational, phase 4 study designed to assess the safety and effectiveness of the fluocinolone acetonide (FAc) implant in all indications in real-world practices in Europe.
Methods: The study included data collected prospectively and retrospectively. Patients receiving FAc implants between 2013 and 2017 were included and monitored until the last patient reached ≥3 years of follow-up. Mean intraocular pressure (IOP) data over the course of the study, along with IOP events, use of IOP-lowering therapy, mean change in visual acuity (VA) and information on supplemental therapy use were analysed post-FAc implantation.
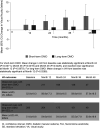
Results: Six hundred and ninety-five eyes from 556 patients, with a mean±SD follow-up of 1150.5±357.36 days, were treated with a FAc implant. 96.7% of eyes had chronic diabetic macular oedema (cDMO). IOP lowering was achieved in 34.5% of eyes using topical agents and 4.3% by surgery. Seventy-three eyes (64.6% of 113 phakic) required cataract surgery during follow-up. Mean VA increased from a baseline of 52.2 letters to 57.1 letters at month 36, with improvement observed up to month 48. Supplementary therapies were given in 43.7% of eyes. When classified by length of cDMO less than or greater than the median duration those with a shorter history experienced greater VA gains than those with a longer history.
Conclusion: This study confirms the favourable, long-term benefit-to-risk profile of the FAc implant in eyes with cDMO, with an additional benefit in patients when this therapy is administered earlier.
Keywords: inflammation; intraocular pressure; macula; treatment medical; vision.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RK - Reports grants from Chengdu Kanghong; grants, personal fees, and non-financial support from Alimera, Bayer, Novartis and Roche, personal fees and non-financial support from Allergan outside the submitted work. TP—speaker honoraria and advisory board member from Alimera Sciences, Allergan, Bayer, Novartis, Boehringer-Ingelheim and Roche. FK—reports a commercial relationship with Alimera Sciences. SRT—reports financial relationships with GlaxoSmithKline and Novartis, and speaker honoraria, advisory boards and travel grants from Alimera Sciences, Allergan, Bayer, GlaxoSmithKline, Novartis and Santen. JPCdS—reports financial support from Alcon, Alimera Sciences and Novartis. LH—statistical consultant to Genentech, Recens Medical, Polyphotonix, and Alimera Sciences. CB—advisory board member for Alimera Sciences, Bayer Novartis, Roche, Janssen, Boehringer-Ingelheim. UC—a speaker and advisory board member for Alimera Sciences, an advisory board member for Allergan, Bayer, Novartis and Roche, and has received grants from Bayer, Novartis and Roche.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical