The MAP3Ks DLK and LZK Direct Diverse Responses to Axon Damage in Zebrafish Peripheral Neurons
- PMID: 35840323
- PMCID: PMC9374156
- DOI: 10.1523/JNEUROSCI.1395-21.2022
The MAP3Ks DLK and LZK Direct Diverse Responses to Axon Damage in Zebrafish Peripheral Neurons
Abstract
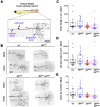
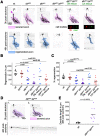
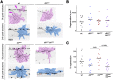
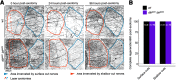
Mitogen-activated protein kinase kinase kinases (MAP3Ks) dual leucine kinase (DLK) and leucine zipper kinase (LZK) are essential mediators of axon damage responses, but their responses are varied, complex, and incompletely understood. To characterize their functions in axon injury, we generated zebrafish mutants of each gene, labeled motor neurons (MNs) and touch-sensing neurons in live zebrafish, precisely cut their axons with a laser, and assessed the ability of mutant axons to regenerate in larvae, before sex is apparent in zebrafish. DLK and LZK were required redundantly and cell autonomously for axon regeneration in MNs but not in larval Rohon-Beard (RB) or adult dorsal root ganglion (DRG) sensory neurons. Surprisingly, in dlk lzk double mutants, the spared branches of wounded RB axons grew excessively, suggesting that these kinases inhibit regenerative sprouting in damaged axons. Uninjured trigeminal sensory axons also grew excessively in mutants when neighboring neurons were ablated, indicating that these MAP3Ks are general inhibitors of sensory axon growth. These results demonstrate that zebrafish DLK and LZK promote diverse injury responses, depending on the neuronal cell identity and type of axonal injury.SIGNIFICANCE STATEMENT The MAP3Ks DLK and LZK are damage sensors that promote diverse outcomes to neuronal injury, including axon regeneration. Understanding their context-specific functions is a prerequisite to considering these kinases as therapeutic targets. To investigate DLK and LZK cell-type-specific functions, we created zebrafish mutants in each gene. Using mosaic cell labeling and precise laser injury we found that both proteins were required for axon regeneration in motor neurons but, unexpectedly, were not required for axon regeneration in Rohon-Beard or DRG sensory neurons and negatively regulated sprouting in the spared axons of touch-sensing neurons. These findings emphasize that animals have evolved distinct mechanisms to regulate injury site regeneration and collateral sprouting, and identify differential roles for DLK and LZK in these processes.
Keywords: DLK; LZK; axon; regeneration; sprouting; zebrafish.
Copyright © 2022 the authors.
Figures



Similar articles
-
A Critical Role for DLK and LZK in Axonal Repair in the Mammalian Spinal Cord.J Neurosci. 2022 May 4;42(18):3716-3732. doi: 10.1523/JNEUROSCI.2495-21.2022. Epub 2022 Mar 31. J Neurosci. 2022. PMID: 35361703 Free PMC article.
-
Targeted disruption of dual leucine zipper kinase and leucine zipper kinase promotes neuronal survival in a model of diffuse traumatic brain injury.Mol Neurodegener. 2019 Nov 27;14(1):44. doi: 10.1186/s13024-019-0345-1. Mol Neurodegener. 2019. PMID: 31775817 Free PMC article.
-
Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons.Sci Rep. 2016 Aug 11;6:31482. doi: 10.1038/srep31482. Sci Rep. 2016. PMID: 27511108 Free PMC article.
-
Multitasking: Dual Leucine Zipper-Bearing Kinases in Neuronal Development and Stress Management.Annu Rev Cell Dev Biol. 2019 Oct 6;35:501-521. doi: 10.1146/annurev-cellbio-100617-062644. Annu Rev Cell Dev Biol. 2019. PMID: 31590586 Free PMC article. Review.
-
Deciphering the multifunctional role of dual leucine zipper kinase (DLK) and its therapeutic potential in disease.Eur J Med Chem. 2023 Jul 5;255:115404. doi: 10.1016/j.ejmech.2023.115404. Epub 2023 Apr 20. Eur J Med Chem. 2023. PMID: 37098296 Review.
Cited by
-
DLK signaling in axotomized neurons triggers complement activation and loss of upstream synapses.Cell Rep. 2024 Feb 27;43(2):113801. doi: 10.1016/j.celrep.2024.113801. Epub 2024 Feb 14. Cell Rep. 2024. PMID: 38363678 Free PMC article.
-
Voltage-gated calcium channels act upstream of adenylyl cyclase Ac78C to promote timely initiation of dendrite regeneration.PLoS Genet. 2024 Aug 26;20(8):e1011388. doi: 10.1371/journal.pgen.1011388. eCollection 2024 Aug. PLoS Genet. 2024. PMID: 39186815 Free PMC article.
-
Dendrite regeneration in the vertebrate spinal cord.Dev Biol. 2022 Aug;488:114-119. doi: 10.1016/j.ydbio.2022.05.014. Epub 2022 May 27. Dev Biol. 2022. PMID: 35644253 Free PMC article.
-
Ciliated sensory neurons can regenerate axons after complete axon removal.J Exp Biol. 2023 Jun 15;226(12):jeb245717. doi: 10.1242/jeb.245717. Epub 2023 Jun 21. J Exp Biol. 2023. PMID: 37212026 Free PMC article.
-
Experimental study on the repair of peripheral nerve injuries via simultaneously coapting the proximal and distal ends of peripheral nerves to the side of nearby intact nerves.Front Neurol. 2023 Apr 6;14:1088983. doi: 10.3389/fneur.2023.1088983. eCollection 2023. Front Neurol. 2023. PMID: 37090979 Free PMC article.
References
-
- Beckers A, Van Dyck A, Bollaerts I, Van Houcke J, Lefevere E, Andries L, Agostinone J, Van Hove I, Di Polo A, Lemmens K, Moons L (2019) An antagonistic axon-dendrite interplay enables efficient neuronal repair in the adult zebrafish central nervous system. Mol Neurobiol 56:3175–3192. 10.1007/s12035-018-1292-5 - DOI - PubMed
-
- Chen M, Geoffroy CG, Meves JM, Narang A, Li Y, Nguyen MT, Khai VS, Kong X, Steinke CL, Carolino KI, Elzière L, Goldberg MP, Jin Y, Zheng B (2018) Leucine zipper-bearing kinase is a critical regulator of astrocyte reactivity in the adult mammalian CNS. Cell Rep 22:3587–3597. 10.1016/j.celrep.2018.02.102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
