Contingent Amygdala Inputs Trigger Heterosynaptic LTP at Hippocampus-To-Accumbens Synapses
- PMID: 35840324
- PMCID: PMC9410749
- DOI: 10.1523/JNEUROSCI.0838-22.2022
Contingent Amygdala Inputs Trigger Heterosynaptic LTP at Hippocampus-To-Accumbens Synapses
Abstract
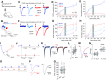
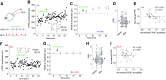
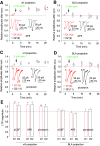
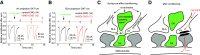
The nucleus accumbens shell (NAcSh) is a key brain region where environmental cues acquire incentive salience to reinforce motivated behaviors. Principal medium spiny neurons (MSNs) in the NAcSh receive extensive glutamatergic projections from limbic regions, among which, the ventral hippocampus (vH) transmits information enriched in contextual cues, and the basolateral amygdala (BLA) encodes real-time arousing states. The vH and BLA project convergently to NAcSh MSNs, both activated in a time-locked manner on a cue-conditioned motivational action. In brain slices prepared from male and female mice, we show that co-activation of the two projections induces long-term potentiation (LTP) at vH-to-NAcSh synapses without affecting BLA-to-NAcSh synapses, revealing a heterosynaptic mechanism through which BLA signals persistently increase the temporally contingent vH-to-NAcSh transmission. Furthermore, this LTP is more prominent in dopamine D1 receptor-expressing (D1) MSNs than D2 MSNs and can be prevented by inhibition of either D1 receptors or dopaminergic terminals in NAcSh. This heterosynaptic LTP may provide a dopamine-guided mechanism through which vH-encoded cue inputs that are contingent to BLA activation acquire increased circuit representation to reinforce behavior.SIGNIFICANCE STATEMENT In motivated behaviors, environmental cues associated with arousing stimuli acquire increased incentive salience, processes mediated in part by the nucleus accumbens (NAc). NAc principal neurons receive glutamatergic projections from the ventral hippocampus (vH) and basolateral amygdala (BLA), which transmit information encoding contextual cues and affective states, respectively. Our results show that co-activation of the two projections induces long-term potentiation (LTP) at vH-to-NAc synapses without affecting BLA-to-NAc synapses, revealing a heterosynaptic mechanism through which BLA signals potentiate the temporally contingent vH-to-NAc transmission. Furthermore, this LTP is prevented by inhibition of either D1 receptors or dopaminergic axons. This heterosynaptic LTP may provide a dopamine-guided mechanism through which vH-encoded cue inputs that are contingent to BLA activation acquire increased circuit representation to reinforce behavior.
Keywords: LTP; amygdala; dopamine; heterosynaptic; hippocampus; nucleus accumbens.
Copyright © 2022 the authors.
Figures


Similar articles
-
Cell-Type- and Endocannabinoid-Specific Synapse Connectivity in the Adult Nucleus Accumbens Core.J Neurosci. 2020 Jan 29;40(5):1028-1041. doi: 10.1523/JNEUROSCI.1100-19.2019. Epub 2019 Dec 12. J Neurosci. 2020. PMID: 31831522 Free PMC article.
-
Cortical and Thalamic Interaction with Amygdala-to-Accumbens Synapses.J Neurosci. 2020 Sep 9;40(37):7119-7132. doi: 10.1523/JNEUROSCI.1121-20.2020. Epub 2020 Aug 6. J Neurosci. 2020. PMID: 32763909 Free PMC article.
-
Excitatory Projections from the Prefrontal Cortex to Nucleus Accumbens Core D1-MSNs and κ Opioid Receptor Modulate Itch-Related Scratching Behaviors.J Neurosci. 2023 Feb 22;43(8):1334-1347. doi: 10.1523/JNEUROSCI.1359-22.2023. Epub 2023 Jan 18. J Neurosci. 2023. PMID: 36653189 Free PMC article.
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens.Mol Psychiatry. 2022 Jan;27(1):669-686. doi: 10.1038/s41380-021-01112-2. Epub 2021 May 7. Mol Psychiatry. 2022. PMID: 33963288 Free PMC article. Review.
-
From valence encoding to motivated behavior: A focus on the nucleus accumbens circuitry.Neurosci Biobehav Rev. 2025 May;172:106125. doi: 10.1016/j.neubiorev.2025.106125. Epub 2025 Mar 26. Neurosci Biobehav Rev. 2025. PMID: 40154653 Review.
Cited by
-
Synaptic sensitization in the anterior cingulate cortex sustains the consciousness of pain via synchronized oscillating electromagnetic waves.Front Hum Neurosci. 2024 Sep 11;18:1462211. doi: 10.3389/fnhum.2024.1462211. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39323956 Free PMC article.
-
Cue- versus reward-encoding basolateral amygdala projections to nucleus accumbens.Elife. 2023 Nov 14;12:e89766. doi: 10.7554/eLife.89766. Elife. 2023. PMID: 37963179 Free PMC article.
-
Specializations in Amygdalar and Hippocampal Innervation of the Primate Nucleus Accumbens Shell.J Neurosci. 2025 Jun 11;45(24):e2425242025. doi: 10.1523/JNEUROSCI.2425-24.2025. J Neurosci. 2025. PMID: 40374561
-
A theory of the neural mechanisms underlying negative cognitive bias in major depression.Front Psychiatry. 2024 Mar 12;15:1348474. doi: 10.3389/fpsyt.2024.1348474. eCollection 2024. Front Psychiatry. 2024. PMID: 38532986 Free PMC article.
-
Heterosynaptic interactions between dorsal and ventral hippocampus in individual medium spiny neurons of the nucleus accumbens ventromedial shell.bioRxiv [Preprint]. 2025 Jun 23:2025.06.23.661109. doi: 10.1101/2025.06.23.661109. bioRxiv. 2025. PMID: 40666985 Free PMC article. Preprint.
References
-
- Akopian G, Crawford C, Beal MF, Cappelletti M, Jakowec MW, Petzinger GM, Zheng L, Gheorghe SL, Reichel CM, Chow R, Walsh JP (2008) Decreased striatal dopamine release underlies increased expression of long-term synaptic potentiation at corticostriatal synapses 24 h after 3-nitropropionic-acid-induced chemical hypoxia. J Neurosci 28:9585–9597. 10.1523/JNEUROSCI.5698-07.2008 - DOI - PMC - PubMed
-
- Allichon MC, Ortiz V, Pousinha P, Andrianarivelo A, Petitbon A, Heck N, Trifilieff P, Barik J, Vanhoutte P (2021) Cell-type-specific adaptions in striatal medium-sized spiny neurons and their roles in behavioral responses to drugs of abuse. Front Synaptic Neurosci 13:799274. 10.3389/fnsyn.2021.799274 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
