Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B
- PMID: 35840551
- PMCID: PMC9287316
- DOI: 10.1038/s41467-022-31486-y
Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B
Abstract
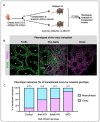
Fate determination and maintenance of fetal testes in most mammals occur cell autonomously as a result of the action of key transcription factors in Sertoli cells. However, the cases of freemartin, where an XX twin develops testis structures under the influence of an XY twin, imply that hormonal factor(s) from the XY embryo contribute to sex reversal of the XX twin. Here we show that in mouse XY embryos, Sertoli cell-derived anti-Mullerian hormone (AMH) and activin B together maintain Sertoli cell identity. Sertoli cells in the gonadal poles of XY embryos lacking both AMH and activin B transdifferentiate into their female counterpart granulosa cells, leading to ovotestis formation. The ovotestes remain to adulthood and produce both sperm and oocytes, although there are few of the former and the latter fail to mature. Finally, the ability of XY mice to masculinize ovaries is lost in the absence of these two factors. These results provide insight into fate maintenance of fetal testes through the action of putative freemartin factors.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
From Enrico Sertoli to freemartinism: the many phases of the master testis-determining cell†.Biol Reprod. 2023 Jun 9;108(6):866-870. doi: 10.1093/biolre/ioad037. Biol Reprod. 2023. PMID: 36951956 Free PMC article. Review.
-
Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion.Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10526-31. doi: 10.1073/pnas.1000318107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498064 Free PMC article.
-
Sox8 and Sox9 act redundantly for ovarian-to-testicular fate reprogramming in the absence of R-spondin1 in mouse sex reversals.Elife. 2020 May 26;9:e53972. doi: 10.7554/eLife.53972. Elife. 2020. PMID: 32450947 Free PMC article.
-
Proteolytic processing of anti-Müllerian hormone differs between human fetal testes and adult ovaries.Mol Hum Reprod. 2015 Jul;21(7):571-82. doi: 10.1093/molehr/gav024. Epub 2015 Apr 28. Mol Hum Reprod. 2015. PMID: 25920489
-
AMH and AMHR2 mutations: A spectrum of reproductive phenotypes across vertebrate species.Dev Biol. 2019 Nov 1;455(1):1-9. doi: 10.1016/j.ydbio.2019.07.006. Epub 2019 Jul 10. Dev Biol. 2019. PMID: 31301298 Free PMC article. Review.
Cited by
-
Inefficient Sox9 upregulation and absence of Rspo1 repression lead to sex reversal in the B6.XYTIR mouse gonad†.Biol Reprod. 2024 May 9;110(5):985-999. doi: 10.1093/biolre/ioae018. Biol Reprod. 2024. PMID: 38376238 Free PMC article.
-
FOXL2 interaction with different binding partners regulates the dynamics of ovarian development.Sci Adv. 2024 Mar 22;10(12):eadl0788. doi: 10.1126/sciadv.adl0788. Epub 2024 Mar 22. Sci Adv. 2024. PMID: 38517962 Free PMC article.
-
Temporal maturation of Sertoli cells during the establishment of the cycle of the seminiferous epithelium†.Biol Reprod. 2024 Oct 14;111(4):959-974. doi: 10.1093/biolre/ioae115. Biol Reprod. 2024. PMID: 39077996 Free PMC article.
-
Postnatal Ovarian Transdifferentiation in the Absence of Estrogen Receptor Signaling Is Dependent on Genetic Background.Endocrinology. 2024 Nov 26;166(1):bqae157. doi: 10.1210/endocr/bqae157. Endocrinology. 2024. PMID: 39576259 Free PMC article.
-
Partial male-to-female reprogramming of mouse fetal testis by Sertoli cell ablation.Development. 2023 Jul 15;150(14):dev201660. doi: 10.1242/dev.201660. Epub 2023 Jul 17. Development. 2023. PMID: 37376880 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

