Hepatic non-parenchymal S100A9-TLR4-mTORC1 axis normalizes diabetic ketogenesis
- PMID: 35840613
- PMCID: PMC9287425
- DOI: 10.1038/s41467-022-31803-5
Hepatic non-parenchymal S100A9-TLR4-mTORC1 axis normalizes diabetic ketogenesis
Abstract
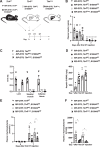
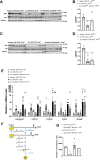
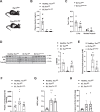
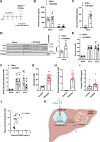
Unrestrained ketogenesis leads to life-threatening ketoacidosis whose incidence is high in patients with diabetes. While insulin therapy reduces ketogenesis this approach is sub-optimal. Here, we report an insulin-independent pathway able to normalize diabetic ketogenesis. By generating insulin deficient male mice lacking or re-expressing Toll-Like Receptor 4 (TLR4) only in liver or hepatocytes, we demonstrate that hepatic TLR4 in non-parenchymal cells mediates the ketogenesis-suppressing action of S100A9. Mechanistically, S100A9 acts extracellularly to activate the mechanistic target of rapamycin complex 1 (mTORC1) in a TLR4-dependent manner. Accordingly, hepatic-restricted but not hepatocyte-restricted loss of Tuberous Sclerosis Complex 1 (TSC1, an mTORC1 inhibitor) corrects insulin-deficiency-induced hyperketonemia. Therapeutically, recombinant S100A9 administration restrains ketogenesis and improves hyperglycemia without causing hypoglycemia in diabetic mice. Also, circulating S100A9 in patients with ketoacidosis is only marginally increased hence unveiling a window of opportunity to pharmacologically augment S100A9 for preventing unrestrained ketogenesis. In summary, our findings reveal the hepatic S100A9-TLR4-mTORC1 axis in non-parenchymal cells as a promising therapeutic target for restraining diabetic ketogenesis.
© 2022. The Author(s).
Conflict of interest statement
G.R. and R.C. are inventors on patent applications related to S100A9 proteins. All other authors declare no competing interests.
Figures



Similar articles
-
S100A9 exerts insulin-independent antidiabetic and anti-inflammatory effects.Sci Adv. 2024 Jan 5;10(1):eadj4686. doi: 10.1126/sciadv.adj4686. Epub 2024 Jan 3. Sci Adv. 2024. PMID: 38170783 Free PMC article.
-
S100A9 extends lifespan in insulin deficiency.Nat Commun. 2019 Aug 7;10(1):3545. doi: 10.1038/s41467-019-11498-x. Nat Commun. 2019. PMID: 31391467 Free PMC article.
-
mTORC1 controls fasting-induced ketogenesis and its modulation by ageing.Nature. 2010 Dec 23;468(7327):1100-4. doi: 10.1038/nature09584. Nature. 2010. PMID: 21179166
-
Roles for insulin and glucagon in the development of ruminant ketosis -- a review.Can Vet J. 1979 May;20(5):121-6. Can Vet J. 1979. PMID: 385136 Free PMC article. Review.
-
Hepatic Mediators of Lipid Metabolism and Ketogenesis: Focus on Fatty Liver and Diabetes.Curr Diabetes Rev. 2021;17(7):e110320187539. doi: 10.2174/1573399816999201103141216. Curr Diabetes Rev. 2021. PMID: 33143628 Review.
Cited by
-
Spontaneous and pharmacologically induced hypothermia protect mice against endotoxic shock.Br J Pharmacol. 2025 Jun;182(12):2621-2641. doi: 10.1111/bph.70000. Epub 2025 Feb 23. Br J Pharmacol. 2025. PMID: 39987925 Free PMC article.
-
mTOR: Its Critical Role in Metabolic Diseases, Cancer, and the Aging Process.Int J Mol Sci. 2024 Jun 2;25(11):6141. doi: 10.3390/ijms25116141. Int J Mol Sci. 2024. PMID: 38892329 Free PMC article. Review.
-
S100A9 exerts insulin-independent antidiabetic and anti-inflammatory effects.Sci Adv. 2024 Jan 5;10(1):eadj4686. doi: 10.1126/sciadv.adj4686. Epub 2024 Jan 3. Sci Adv. 2024. PMID: 38170783 Free PMC article.
-
Atractylenolide-I restore intestinal barrier function by targeting the S100A9/AMPK/mTOR signaling pathway.Front Pharmacol. 2025 Mar 24;16:1530109. doi: 10.3389/fphar.2025.1530109. eCollection 2025. Front Pharmacol. 2025. PMID: 40196359 Free PMC article.
-
Polyploidy and mTOR signaling: a possible molecular link.Cell Commun Signal. 2024 Mar 27;22(1):196. doi: 10.1186/s12964-024-01526-9. Cell Commun Signal. 2024. PMID: 38539200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

