Discovery of a novel class of benzimidazoles as highly effective agonists of bone morphogenetic protein (BMP) receptor signaling
- PMID: 35840622
- PMCID: PMC9287337
- DOI: 10.1038/s41598-022-16394-x
Discovery of a novel class of benzimidazoles as highly effective agonists of bone morphogenetic protein (BMP) receptor signaling
Abstract
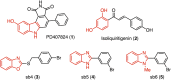
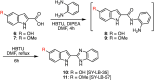
Increasing or restoring Bone Morphogenetic Protein receptor signaling is an effective therapy for conditions such as bone fracture and pulmonary arterial hypertension. However, direct use of recombinant BMPs has encountered significant obstacles. Moreover, synthetic, full agonists of BMP receptor signaling have yet to be identified. Here, we report the discovery of a novel class of indolyl-benzimidazoles, synthesized using a one-pot synthetic methodology, which appear to mimic the biochemical and functional activity of BMPs. The first-in-series compounds, SY-LB-35 and SY-LB-57, stimulated significant increases in cell number and cell viability in the C2C12 myoblast cell line. Cell cycle analysis revealed that these compounds induced a shift toward proliferative phases. SY-LB-35 and SY-LB-57 stimulated canonical Smad and non-canonical PI3K/Akt, ERK, p38 and JNK intracellular signaling pathways, similar to BMP2-stimulated responses. Importantly, increases in Smad phosphorylation and cell viability were dependent on type I BMP receptor activity. Thus, these compounds robustly activate intracellular signaling in a BMP receptor-dependent manner and may signify the first known, full agonists of BMP receptor signaling. Moreover, discovery of small molecule activators of BMP pathways, which can be efficiently formulated and targeted to diseased or damaged areas, could potentially substitute recombinant BMPs for treatment of BMP-related pathologies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Novel Indolyl-Benzimidazole Compounds Promote in vitro Wound Healing and Osteogenic Differentiation of Pluripotent Cells.Front Biosci (Landmark Ed). 2023 Oct 27;28(10):268. doi: 10.31083/j.fbl2810268. Front Biosci (Landmark Ed). 2023. PMID: 37919087
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
High-throughput measurements of bone morphogenetic protein/bone morphogenetic protein receptor interactions using biolayer interferometry.Biointerphases. 2021 Jun 8;16(3):031001. doi: 10.1116/6.0000926. Biointerphases. 2021. PMID: 34241280 Free PMC article.
-
BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.Exp Cell Res. 2010 Jan 1;316(1):24-37. doi: 10.1016/j.yexcr.2009.10.010. Epub 2009 Oct 14. Exp Cell Res. 2010. PMID: 19835871
-
BMP Signaling Pathway in Dentin Development and Diseases.Cells. 2022 Jul 16;11(14):2216. doi: 10.3390/cells11142216. Cells. 2022. PMID: 35883659 Free PMC article. Review.
Cited by
-
Therapeutic Intervention of Neuroinflammatory Alzheimer Disease Model by Inhibition of Classical Complement Pathway with the Use of Anti-C1r Loaded Exosomes.Res Sq [Preprint]. 2023 Oct 18:rs.3.rs-3399248. doi: 10.21203/rs.3.rs-3399248/v1. Res Sq. 2023. PMID: 37886595 Free PMC article. Preprint.
-
Small-Molecule Probes as Pharmacological Tools for the Bone Morphogenetic Protein Signaling Pathway.ACS Pharmacol Transl Sci. 2023 Oct 27;6(11):1574-1599. doi: 10.1021/acsptsci.3c00170. eCollection 2023 Nov 10. ACS Pharmacol Transl Sci. 2023. PMID: 37974621 Free PMC article. Review.
-
Pharmacological modulation of stem cells signaling pathway for therapeutic applications.Stem Cell Res Ther. 2025 Jul 1;16(1):327. doi: 10.1186/s13287-025-04438-8. Stem Cell Res Ther. 2025. PMID: 40598543 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

