Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial
- PMID: 35840747
- PMCID: PMC9532243
- DOI: 10.1038/s41409-022-01754-y
Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial
Abstract
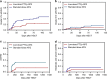
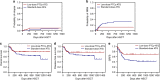
The most widely used regimens of graft-versus-host disease (GVHD) prophylaxis in HLA-matched unrelated donor peripheral blood stem cell transplantation (MUD-PBSCT) are based on anti-thymocyte globulin (ATG) or post-transplant cyclophosphamide (PTCy). To improve the efficiency of GVHD prophylaxis, a novel regimen, composed of low-dose PTCy (20 mg/kg on day +3 and +4) and low-dose ATG (6 mg/kg), was evaluted in patients with hematological malignancies ungoing 10/10 HLA MUD-PBSCT in first remission (CR1). In our prospective, multicenter study, 104 patients were randomly assigned one-to-one to low-dose PTCy-ATG (n = 53) or standard-dose ATG (10 mg/kg, n = 51). Both the cumulative incidences (CIs) of grade II-IV acute GVHD (aGVHD) and chronic GVHD (cGVHD) at 2 years in low-dose PTCy-ATG cohort were significantly reduced (24.5% vs. 47.1%; P = 0.017; 14.1% vs. 33.3%; P = 0.013). The CI of non-relapse-mortality (NRM) was much lower (13.2% vs. 34.5%; P = 0.049) and GVHD-free, relapse-free survival (GRFS) was significantly improved at 2 years in low-dose PTCy-ATG arm (67.3% vs 42.3%; P = 0.032). The low-dose PTCy-ATG based GVHD prophylaxis is a promising strategy for patients in CR1 after 10/10 HLA MUD-PBSCT.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Outcomes of Antithymocyte Globulin-Post-Transplantation Cyclophosphamide-Cyclosporine-Based versus Antithymocyte Globulin-Based Prophylaxis for 10/10 HLA-Matched Unrelated Donor Allogeneic Hematopoietic Cell Transplantation.Transplant Cell Ther. 2024 May;30(5):536.e1-536.e13. doi: 10.1016/j.jtct.2024.01.075. Epub 2024 Jan 27. Transplant Cell Ther. 2024. PMID: 38281592
-
Reduced-dose post-transplant cyclophosphamide plus low-dose post-transplant anti-thymocyte globulin as graft-versus-host disease prophylaxis with fludarabine-busulfan-cytarabine conditioning in haploidentical peripheral blood stem cell transplantation: A multicentre, randomized controlled clinical trial.Br J Haematol. 2023 Jan;200(2):210-221. doi: 10.1111/bjh.18483. Epub 2022 Oct 6. Br J Haematol. 2023. PMID: 36200642 Clinical Trial.
-
Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors.J Hematol Oncol. 2020 Jul 3;13(1):87. doi: 10.1186/s13045-020-00923-0. J Hematol Oncol. 2020. PMID: 32620146 Free PMC article.
-
Combination of Anti-thymocyte Globulin with Post-transplant Cyclophosphamide for GVHD Prophylaxis in Patients Undergoing Haploidentical Hematopoietic Stem Cell Transplantation: Systematic Review and Meta-analysis.Transplant Cell Ther. 2025 Jan;31(1):32.e1-32.e15. doi: 10.1016/j.jtct.2024.07.017. Epub 2024 Jul 29. Transplant Cell Ther. 2025. PMID: 39084262
-
Post-transplant cyclophosphamide versus antithymocyte globulin in allogeneic hematopoietic cell transplantation: a meta-analysis.Ann Hematol. 2021 Feb;100(2):529-540. doi: 10.1007/s00277-021-04399-x. Epub 2021 Jan 8. Ann Hematol. 2021. PMID: 33420575
Cited by
-
Evaluating the Impact of Post-Transplant Cyclophosphamide and Anti-Thymocyte Globulin on CMV Reactivation Following Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Literature Review.J Clin Med. 2023 Dec 18;12(24):7765. doi: 10.3390/jcm12247765. J Clin Med. 2023. PMID: 38137835 Free PMC article. Review.
-
Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for Fanconi anemia with/without anti-thymocyte globulin.Blood Cell Ther. 2024 Aug 9;7(3):95-100. doi: 10.31547/bct-2024-006. eCollection 2024 Aug 25. Blood Cell Ther. 2024. PMID: 39263620 Free PMC article.
-
Reduced Dose of Post-Transplant Cyclophosphamide with Tacrolimus for the Prevention of Graft-versus-Host Disease in HLA-Matched Donor Peripheral Blood Stem Cell Transplants: A Prospective Pilot Study.Cancers (Basel). 2024 Jul 17;16(14):2567. doi: 10.3390/cancers16142567. Cancers (Basel). 2024. PMID: 39061206 Free PMC article.
-
The role of allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: A review.Front Oncol. 2023 Jan 18;12:1105779. doi: 10.3389/fonc.2022.1105779. eCollection 2022. Front Oncol. 2023. PMID: 36741737 Free PMC article. Review.
-
Anti-T lymphocyte globulin plus posttransplant cyclophosphamide 25 mg/kg versus posttransplant cyclophosphamide 50 mg/kg in patients with acute leukemias.Bone Marrow Transplant. 2025 Jun;60(6):864-872. doi: 10.1038/s41409-025-02564-8. Epub 2025 Apr 13. Bone Marrow Transplant. 2025. PMID: 40223010 Free PMC article.
References
-
- Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73. doi: 10.1016/s1470-2045(15)00462-3. - DOI - PubMed
-
- Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4:e293–e301. doi: 10.1016/s2352-3026(17)30081-9. - DOI - PubMed
-
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167. doi: 10.1016/s2352-3026(19)30256-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

