Flavaspidic acid BB combined with mupirocin improves its anti-bacterial and anti-biofilm activities against Staphylococcus epidermidis
- PMID: 35840879
- PMCID: PMC9284735
- DOI: 10.1186/s12866-022-02578-y
Flavaspidic acid BB combined with mupirocin improves its anti-bacterial and anti-biofilm activities against Staphylococcus epidermidis
Abstract
Background: The increase in drug-resistant opportunistic pathogenic bacteria, especially of antibiotic-resistant Staphylococcus epidermidis (S. epidermidis), has led to difficulties in the treatment of skin and soft tissue infections (SSTI). The major reason for bacterial resistance is the formation of bacterial biofilm. Here, we report a promising combination therapy of flavaspidic acid BB (BB) and mupirocin, which can effectively eradicate the biofilm of S. epidermidis and eliminate its drug resistance.
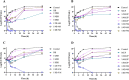
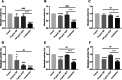
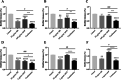
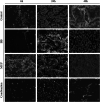
Result: The susceptibility test showed that the combination of BB and mupirocin has good antibacterial and antibiofilm activities, and the fractional inhibitory concentration index (FICI) of BB combined with mupirocin was 0.51 ± 0.00 ~ 0.75 ± 0.05, showing synergistic effect. Moreover, the time-kill curve assay results indicated that the combination of drugs can effectively inhibit the planktonic S. epidermidis. After drugs treatment, the drug-combination showed significantly inhibitory effects on the metabolic activity and total biomass in each stage of biofilm formation. The synergistic effect is likely related to the adhesion between bacteria, which is confirmed by field emission scanning electron microscope. And the expression level of aap, sarA and agrA genes were detected by real-time quantitative PCR (qRT-PCR).
Conclusion: Our study provides the experimental data for the use of BB for the clinical treatment of skin infections and further demonstrate the potential of BB as a novel biofilm inhibitor.
Keywords: Biofilm; Combination therapy; Flavaspidic acid BB; Staphylococcus epidermidis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Antibacterial activity and antibacterial mechanism of flavaspidic acid BB against Staphylococcus haemelyticus.BMC Microbiol. 2023 Sep 29;23(1):276. doi: 10.1186/s12866-023-02997-5. BMC Microbiol. 2023. PMID: 37773054 Free PMC article.
-
Antibacterial and anti-biofilm activities of Disaspidin BB against Staphylococcus epidermidis.Front Microbiol. 2023 Jan 19;14:999449. doi: 10.3389/fmicb.2023.999449. eCollection 2023. Front Microbiol. 2023. PMID: 36744091 Free PMC article.
-
Mupirocin at Subinhibitory Concentrations Induces Biofilm Formation in Staphylococcus aureus.Microb Drug Resist. 2018 Nov;24(9):1249-1258. doi: 10.1089/mdr.2017.0290. Epub 2018 Mar 14. Microb Drug Resist. 2018. PMID: 29653478
-
Antibiofilm activity of three Actinomycete strains against Staphylococcus epidermidis.Lett Appl Microbiol. 2019 Jan;68(1):73-80. doi: 10.1111/lam.13087. Epub 2018 Nov 22. Lett Appl Microbiol. 2019. PMID: 30338533 Review.
-
Clinical relevance of mupirocin resistance in Staphylococcus aureus.J Hosp Infect. 2013 Dec;85(4):249-56. doi: 10.1016/j.jhin.2013.09.006. Epub 2013 Sep 21. J Hosp Infect. 2013. PMID: 24144552 Review.
Cited by
-
Effects of honokiol combined with resveratrol on bacteria responsible for oral malodor and their biofilm.J Oral Microbiol. 2024 Jun 9;16(1):2361402. doi: 10.1080/20002297.2024.2361402. eCollection 2024. J Oral Microbiol. 2024. PMID: 38860120 Free PMC article.
-
Improving Activity of New Arylurea Agents against Multidrug-Resistant and Biofilm-Producing Staphylococcus epidermidis.ACS Med Chem Lett. 2024 Feb 5;15(3):369-375. doi: 10.1021/acsmedchemlett.3c00536. eCollection 2024 Mar 14. ACS Med Chem Lett. 2024. PMID: 38505856 Free PMC article.
-
Antibacterial activity and antibacterial mechanism of flavaspidic acid BB against Staphylococcus haemelyticus.BMC Microbiol. 2023 Sep 29;23(1):276. doi: 10.1186/s12866-023-02997-5. BMC Microbiol. 2023. PMID: 37773054 Free PMC article.
References
-
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52. doi: 10.1093/cid/ciu444. - DOI - PubMed
-
- Llopis F, González-Castillo J, Julián-Jiménez A, Ferré C, Gamazo-Río JJ, Martínez M, INFURG-SEMES Group Review of 1.250 episodes of skin and soft tissue infections attended at 49 hospital emergency departments. Rev Esp Quimioter. 2014;27(2):115–121. - PubMed
-
- PD Vos GM Garrity D Jones NR Krieg W Ludwig FA Rainey et al 2009 Bergey’s manual of systematic bacteriology 38 4 443 491 10.1007/978-0-387-68489-5
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

