Overexpression VaPYL9 improves cold tolerance in tomato by regulating key genes in hormone signaling and antioxidant enzyme
- PMID: 35840891
- PMCID: PMC9284830
- DOI: 10.1186/s12870-022-03704-8
Overexpression VaPYL9 improves cold tolerance in tomato by regulating key genes in hormone signaling and antioxidant enzyme
Abstract
Background: Abscisic acid (ABA) has been reported in controlling plant growth and development, and particularly dominates a role in resistance to abiotic stress. The Pyrabactin Resistance1/PYR1-Like /Regulatory Components of ABA receptors (PYR1/PYL/RCAR) gene family, of which the PYL9 is a positive regulator related to stress response in ABA signaling transduction. Although the family has been identified in grape, detailed VaPYL9 function in cold stress remains unknown.
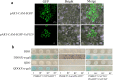
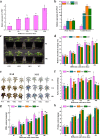
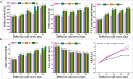
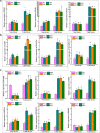
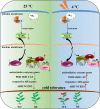
Results: In order to explore the cold tolerance mechanism in grape, VaPYL9 was cloned from Vitis amurensis. The subcellular localization showed that VaPYL9 was mainly expressed in the plasma membrane. Yeast two-hybrid (Y2H) showed VaPCMT might be a potential interaction protein of VaPYL9. Through the overexpression of VaPYL9 in tomatoes, results indicated transgenic plants had higher antioxidant enzyme activities and proline content, lower malondialdehyde (MDA) and H2O2 content, and improving the ability to scavenge reactive oxygen species than wild-type (WT). Additionally, ABA content and the ratio of ABA/IAA kept a higher level than WT. Quantitative real-time PCR (qRT-PCR) showed that VaPYL9, SlNCED3, SlABI5, and antioxidant enzyme genes (POD, SOD, CAT) were up-regulated in transgenic tomatoes. Transcriptome sequencing (RNA-seq) found that VaPYL9 overexpression caused the upregulation of key genes PYR/PYL, PYL4, MAPK17/18, and WRKY in transgenic tomatoes under cold stress.
Conclusion: Overexpression VaPYL9 enhances cold resistance of transgenic tomatoes mediated by improving antioxidant enzymes activity, reducing membrane damages, and regulating key genes in plant hormones signaling and antioxidant enzymes.
Keywords: Abscisic acid; Cold stress; Expression analysis; Overexpression VaPYL9; RNA-seq.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Gokul A, Niekerk LA, Carelse MF, Keyster M. Transgenic technology for efficient abiotic stress tolerance in plants (Chapter 5). In: Kiran U, Abdin M, Kamaluddin, editors. Transgenic Technology Based Value Addition in Plant Biotechnology. Amsterdam: Elsevier; 2020. P. 95-122. 10.1016/b978-0-12-818632-9.00005-8.
-
- Lata C, Shivhare R. Engineering cereal crops for enhanced abiotic stress tolerance. P Indian Natl Sci Ac. 2021;87(1):6–83. doi: 10.1007/s43538-021-00006-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

