Ginsenoside Rg3 induces apoptosis and inhibits proliferation by down-regulating TIGAR in rats with gastric precancerous lesions
- PMID: 35840932
- PMCID: PMC9284801
- DOI: 10.1186/s12906-022-03669-z
Ginsenoside Rg3 induces apoptosis and inhibits proliferation by down-regulating TIGAR in rats with gastric precancerous lesions
Erratum in
-
Correction: Ginsenoside Rg3 induces apoptosis and inhibits proliferation by down-regulating TIGAR in rats with gastric precancerous lesions.BMC Complement Med Ther. 2023 Feb 27;23(1):65. doi: 10.1186/s12906-023-03882-4. BMC Complement Med Ther. 2023. PMID: 36850010 Free PMC article. No abstract available.
Abstract
Background: Ginsenoside Rg3 (GRg3) is one of the main active ingredients in Chinese ginseng extract and has various biological effects, such as immune-enhancing, antitumour, antiangiogenic, immunomodulatory and anti-inflammatory effects. This study aimed to investigate the therapeutic effect of GRg3 on gastric precancerous lesion (GPL) induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and the potential mechanism of action.
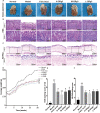
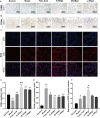
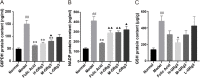
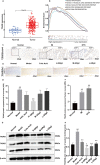
Methods: The MNNG-ammonia composite modelling method was used to establish a rat model of GPL. Histopathological changes in the rat gastric mucosa were observed by pathological analysis using haematoxylin-eosin staining to assess the success rate of the composite modelling method. Alcian blue-periodic acid Schiff staining was used to observe intestinal metaplasia in the rat gastric mucosa. Apoptosis was detected in rat gastric mucosal cells by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling staining. The production level of reactive oxygen species (ROS) was determined by the dihydroethidium fluorescent probe method, and that of TP53-induced glycolysis and apoptosis regulator (TIGAR) protein was determined by immunohistochemical staining and western blotting. The production levels of nicotinamide adenine dinucleotide phosphate (NADP) and glucose-6-phosphate dehydrogenase (G6PDH) were determined by an enzyme-linked immunosorbent assay, and that of glutathione (GSH) was determined by microanalysis.
Results: GRg3 significantly alleviated the structural disorganization and cellular heteromorphism in the form of epithelial glands in the gastric mucosa of rats with GPL and retarded the progression of the disease. Overexpression of TIGAR and overproduction of NADP, GSH and G6PDH occurred in the gastric mucosal epithelium of rats with GPL, which in turn led to an increase in the ROS concentration. After treatment with GRg3, the expression of TIGAR and production of NADP, GSH G6PDH decreased, causing a further increase in the concentration of ROS in the gastric mucosal epithelium, which in turn induced apoptosis and played a role in inhibiting the abnormal proliferation and differentiation of gastric mucosal epithelial cells.
Conclusion: Grg3 can induce apoptosis and inhibit cell proliferation in MNNG-induced GPL rats. The mechanism may be related to down-regulating the expression levels of TIGAR and production levels of GSH, NADP and G6PD, and up-regulating the concentration of ROS.
Keywords: Apoptosis; Gastric precancerous lesions; Ginsenoside Rg3; Reactive oxygen species; TP53-induced glycolysis and apoptosis regulator.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

References
MeSH terms
Substances
Grants and funding
- 81804066/National Natural Science Foundation of China
- QNXZ2019017/Xinglin Scholar Research Promotion Project of Chengdu University of TCM
- 21-Y17/"Hundred Talents Program" of the Hospital of Chengdu University of Traditional Chinese Medicine
- 2021MS104/Project of Sichuan Administration of traditional Chi- nese Medicine
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

