Proton export upregulates aerobic glycolysis
- PMID: 35840963
- PMCID: PMC9287933
- DOI: 10.1186/s12915-022-01340-0
Proton export upregulates aerobic glycolysis
Abstract
Introduction: Aggressive cancers commonly ferment glucose to lactic acid at high rates, even in the presence of oxygen. This is known as aerobic glycolysis, or the "Warburg Effect." It is widely assumed that this is a consequence of the upregulation of glycolytic enzymes. Oncogenic drivers can increase the expression of most proteins in the glycolytic pathway, including the terminal step of exporting H+ equivalents from the cytoplasm. Proton exporters maintain an alkaline cytoplasmic pH, which can enhance all glycolytic enzyme activities, even in the absence of oncogene-related expression changes. Based on this observation, we hypothesized that increased uptake and fermentative metabolism of glucose could be driven by the expulsion of H+ equivalents from the cell.
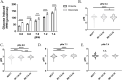
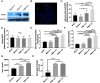
Results: To test this hypothesis, we stably transfected lowly glycolytic MCF-7, U2-OS, and glycolytic HEK293 cells to express proton-exporting systems: either PMA1 (plasma membrane ATPase 1, a yeast H+-ATPase) or CA-IX (carbonic anhydrase 9). The expression of either exporter in vitro enhanced aerobic glycolysis as measured by glucose consumption, lactate production, and extracellular acidification rate. This resulted in an increased intracellular pH, and metabolomic analyses indicated that this was associated with an increased flux of all glycolytic enzymes upstream of pyruvate kinase. These cells also demonstrated increased migratory and invasive phenotypes in vitro, and these were recapitulated in vivo by more aggressive behavior, whereby the acid-producing cells formed higher-grade tumors with higher rates of metastases. Neutralizing tumor acidity with oral buffers reduced the metastatic burden.
Conclusions: Therefore, cancer cells which increase export of H+ equivalents subsequently increase intracellular alkalization, even without oncogenic driver mutations, and this is sufficient to alter cancer metabolism towards an upregulation of aerobic glycolysis, a Warburg phenotype. Overall, we have shown that the traditional understanding of cancer cells favoring glycolysis and the subsequent extracellular acidification is not always linear. Cells which can, independent of metabolism, acidify through proton exporter activity can sufficiently drive their metabolism towards glycolysis providing an important fitness advantage for survival.
Keywords: CA-IX; Cancer; Glycolysis; Metastasis; PMA1; Proton; Warburg; pH.
© 2022. The Author(s).
Conflict of interest statement
RJG is supported by a grant from Helix, Biopharma. The other authors declare that they have no competing interests.
Figures




References
-
- Beketic-Oreskovic L, Ozretic P, Rabbani ZN, Jackson IL, Sarcevic B, Levanat S, Vujaskovic Z. Prognostic significance of carbonic anhydrase IX (CAIX), endoglin (CD105), and 8-hydroxy-2deoxyguanosine (8-OHdG) in breast cancer patients. J Clin Oncol. 2010;28:e21048–e21048. doi: 10.1200/jco.2010.28.15_suppl.e21048. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

