Titanium dioxide and carbon black nanoparticles disrupt neuronal homeostasis via excessive activation of cellular prion protein signaling
- PMID: 35840975
- PMCID: PMC9284759
- DOI: 10.1186/s12989-022-00490-x
Titanium dioxide and carbon black nanoparticles disrupt neuronal homeostasis via excessive activation of cellular prion protein signaling
Abstract
Background: Epidemiological emerging evidence shows that human exposure to some nanosized materials present in the environment would contribute to the onset and/or progression of Alzheimer's disease (AD). The cellular and molecular mechanisms whereby nanoparticles would exert some adverse effects towards neurons and take part in AD pathology are nevertheless unknown.
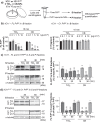
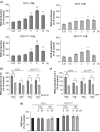
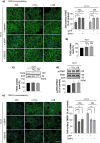
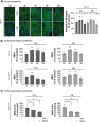
Results: Here, we provide the prime evidence that titanium dioxide (TiO2) and carbon black (CB) nanoparticles (NPs) bind the cellular form of the prion protein (PrPC), a plasma membrane protein well known for its implication in prion diseases and prion-like diseases, such as AD. The interaction between TiO2- or CB-NPs and PrPC at the surface of neuronal cells grown in culture corrupts PrPC signaling function. This triggers PrPC-dependent activation of NADPH oxidase and subsequent production of reactive oxygen species (ROS) that alters redox equilibrium. Through PrPC interaction, NPs also promote the activation of 3-phosphoinositide-dependent kinase 1 (PDK1), which in turn provokes the internalization of the neuroprotective TACE α-secretase. This diverts TACE cleavage activity away from (i) TNFα receptors (TNFR), whose accumulation at the plasma membrane augments the vulnerability of NP-exposed neuronal cells to TNFα -associated inflammation, and (ii) the amyloid precursor protein APP, leading to overproduction of neurotoxic amyloid Aβ40/42 peptides. The silencing of PrPC or the pharmacological inhibition of PDK1 protects neuronal cells from TiO2- and CB-NPs effects regarding ROS production, TNFα hypersensitivity, and Aβ rise. Finally, we show that dysregulation of the PrPC-PDK1-TACE pathway likely occurs in the brain of mice injected with TiO2-NPs by the intra-cerebro-ventricular route as we monitor a rise of TNFR at the cell surface of several groups of neurons located in distinct brain areas.
Conclusion: Our in vitro and in vivo study thus posits for the first time normal cellular prion protein PrPC as being a neuronal receptor of TiO2- and CB-NPs and identifies PrPC-coupled signaling pathways by which those nanoparticles alter redox equilibrium, augment the intrinsic sensitivity of neurons to neuroinflammation, and provoke a rise of Aβ peptides. By identifying signaling cascades dysregulated by TiO2- and CB-NPs in neurons, our data shed light on how human exposure to some NPs might be related to AD.
Keywords: Alzheimer’s disease; Aβ peptides; Nanoneurotoxicity; Nanoparticles; Neuroinflammation; PrPC receptor; Signaling; TNFα receptors.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Kaphle A, Navya PN, Umapathi A, Daima HK. Nanomaterials for agriculture, food and environment: applications, toxicity and regulation. Environ Chem Lett. 2018;16:43–58. doi: 10.1007/s10311-017-0662-y. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

