Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling
- PMID: 35841100
- PMCID: PMC9288088
- DOI: 10.1186/s12974-022-02549-0
Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling
Abstract
Background: In chronic myelogenous leukemia, reciprocal translocation between chromosome 9 and chromosome 22 generates a chimeric protein, Bcr-Abl, that leads to hyperactivity of tyrosine kinase-linked signaling transduction. The therapeutic agent nilotinib inhibits Bcr-Abl/DDR1 and can cross the blood-brain barrier, but its potential impact on neuroinflammatory responses and cognitive function has not been studied in detail.
Methods: The effects of nilotinib in vitro and in vivo were assessed by a combination of RT-PCR, real-time PCR, western blotting, ELISA, immunostaining, and/or subcellular fractionation. In the in vitro experiments, the effects of 200 ng/mL LPS or PBS on BV2 microglial cells, primary microglia or primary astrocytes pre- or post-treated with 5 µM nilotinib or vehicle were evaluated. The in vivo experiments involved wild-type mice administered a 7-day course of daily injections with 20 mg/kg nilotinib (i.p.) or vehicle before injection with 10 mg/kg LPS (i.p.) or PBS.
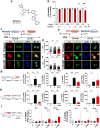
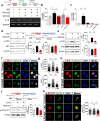
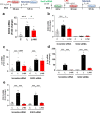
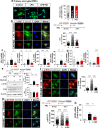
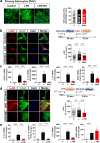
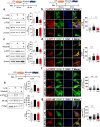
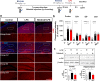
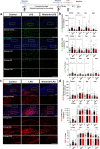
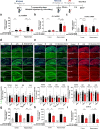
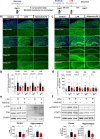
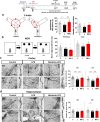
Results: In BV2 microglial cells, pre- and post-treatment with nilotinib altered LPS-induced proinflammatory/anti-inflammatory cytokine mRNA levels by suppressing AKT/P38/SOD2 signaling. Nilotinib treatment also significantly downregulated LPS-stimulated proinflammatory cytokine levels in primary microglia and primary astrocytes by altering P38/STAT3 signaling. Experiments in wild-type mice showed that nilotinib administration affected LPS-mediated microglial/astroglial activation in a brain region-specific manner in vivo. In addition, nilotinib significantly reduced proinflammatory cytokine IL-1β, IL-6 and COX-2 levels and P38/STAT3 signaling in the brain in LPS-treated wild-type mice. Importantly, nilotinib treatment rescued LPS-mediated spatial working memory impairment and cortical dendritic spine number in wild-type mice.
Conclusions: Our results indicate that nilotinib can modulate neuroinflammatory responses and cognitive function in LPS-stimulated wild-type mice.
Keywords: Cognitive function; LPS; Microglia; Nilotinib; SOD2; STAT3; p38.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Pagan FL, Hebron ML, Wilmarth B, Torres-Yaghi Y, Lawler A, Mundel EE, Yusuf N, Starr NJ, Arellano J, Howard HH, et al. Pharmacokinetics and pharmacodynamics of a single dose nilotinib in individuals with Parkinson’s disease. Pharmacol Res Perspect. 2019;7(2):e00470. doi: 10.1002/prp2.470. - DOI - PMC - PubMed
-
- Reinwald M, Schleyer E, Kiewe P, Blau IW, Burmeister T, Pursche S, Neumann M, Notter M, Thiel E, Hofmann WK, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int. 2014;2014:637059. doi: 10.1155/2014/637059. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

