The development and initial validation of the PROMIS®+HF-27 and PROMIS+HF-10 profiles
- PMID: 35841128
- PMCID: PMC9715763
- DOI: 10.1002/ehf2.14061
The development and initial validation of the PROMIS®+HF-27 and PROMIS+HF-10 profiles
Abstract
Aims: Heart failure (HF) is a common and morbid condition impacting multiple health domains. We previously reported the development of the PROMIS®-Plus-HF (PROMIS+HF) profile measure, including universal and HF-specific items. To facilitate use, we developed shorter, PROMIS+HF profiles intended for research and clinical use.
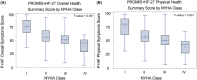
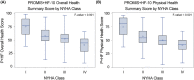
Methods and results: Candidate items were selected based on psychometric properties and symptom range coverage. HF clinicians (n = 43) rated item importance and clinical actionability. Based on these results, we developed the PROMIS+HF-27 and PROMIS+HF-10 profiles with summary scores (0-100) for overall, physical, mental, and social health. In a cross-sectional sample (n = 600), we measured internal consistency reliability (Cronbach's alpha and Spearman-Brown), test-retest reliability (intraclass coefficient; n = 100), known-groups validity via New York Heart Association (NYHA) class, and convergent validity with Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. In a longitudinal sample (n = 75), we evaluated responsiveness of baseline/follow-up scores by calculating mean differences and Cohen's d and comparing with paired t-tests. Internal consistency was good to excellent (α 0.82-0.94) for all PROMIS+HF-27 scores and acceptable to good (α/Spearman-Brown 0.60-0.85) for PROMIS+HF-10 scores. Test-retest intraclass coefficients were acceptable to excellent (0.75-0.97). Both profiles demonstrated known-groups validity for the overall and physical health summary scores based on NYHA class, and convergent validity for nearly all scores compared with KCCQ scores. In the longitudinal sample, we demonstrated responsiveness for PROMIS+HF-27 and PROMIS+HF-10 overall and physical summary scores. For the PROMIS+HF overall summary scores, a group-based increase of 7.6-8.3 points represented a small to medium change (Cohen's d = 0.40-0.42). For the PROMIS+HF physical summary scores, a group-based increase of 5.0-5.9 points represented a small to medium change (Cohen's d = 0.29-0.35).
Conclusions: The PROMIS+HF-27 and PROMIS+HF-10 profiles demonstrated good psychometric characteristics with evidence of responsiveness for overall and physical health. These new measures can facilitate patient-centred research and clinical care, such as improving care quality through symptom monitoring, facilitating shared decision-making, evaluating quality of care, assessing new interventions, and monitoring during the initiation and titration of guideline-directed medical therapy.
Keywords: Health status; Heart failure; Outcomes research; Patient-reported outcomes; Quality of life.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Development and Initial Validation of the PROMIS®-Plus-HF Profile Measure.Circ Heart Fail. 2019 Jun;12(6):e005751. doi: 10.1161/CIRCHEARTFAILURE.118.005751. Epub 2019 Jun 5. Circ Heart Fail. 2019. PMID: 31163985 Free PMC article.
-
Psychometric properties of the Japanese version of the Kansas City Cardiomyopathy Questionnaire in Japanese patients with chronic heart failure.Health Qual Life Outcomes. 2020 Jul 17;18(1):236. doi: 10.1186/s12955-020-01483-0. Health Qual Life Outcomes. 2020. PMID: 32680513 Free PMC article.
-
The PROMISⓇ-Plus-Osteoarthritis of the Knee (OAK) profile measure integrates generic and condition-specific content to enhance relevance and efficiency.J Clin Epidemiol. 2021 Jul;135:158-169. doi: 10.1016/j.jclinepi.2021.03.028. Epub 2021 Apr 9. J Clin Epidemiol. 2021. PMID: 33839241
-
Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction.Circ Heart Fail. 2013 Nov;6(6):1139-46. doi: 10.1161/CIRCHEARTFAILURE.113.000359. Epub 2013 Oct 15. Circ Heart Fail. 2013. PMID: 24130003 Free PMC article.
-
Adding Disease-Specific Concerns to Patient-Reported Outcome Measures [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Apr. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Apr. PMID: 38416860 Free Books & Documents. Review.
Cited by
-
Selecting patient-reported outcome measures: "what" and "for whom".Health Aff Sch. 2024 Mar 27;2(4):qxae038. doi: 10.1093/haschl/qxae038. eCollection 2024 Apr. Health Aff Sch. 2024. PMID: 38756176 Free PMC article.
-
Challenges of Health Data Use in Multidisciplinary Chronic Disease Care: Perspective from Heart Failure Care.J Cardiovasc Dev Dis. 2023 Dec 5;10(12):486. doi: 10.3390/jcdd10120486. J Cardiovasc Dev Dis. 2023. PMID: 38132654 Free PMC article.
References
-
- Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ, American Heart Association Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council . Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013; 127: 2233–2249. - PubMed
-
- Institute of Medicine (US) Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. - PubMed
-
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O'Neill R, Kennedy DL. Patient‐reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007; 10: S125–S137. - PubMed
-
- Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, Filippatos GS, Gheorghiade M, Hernandez AF, Jaarsma T, Koglin J, Konstam M, Kupfer S, Maggioni AP, Mebazaa A, Metra M, Nowack C, Pieske B, Pina IL, Pocock SJ, Ponikowski P, Rosano G, Ruilope LM, Ruschitzka F, Severin T, Solomon S, Stein K, Stockbridge NL, Stough WG, Swedberg K, Tavazzi L, Voors AA, Wasserman SM, Woehrle H, Zalewski A, McMurray JJ. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. 2013; 15: 1082–1094. - PubMed
-
- Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I, Kirchhof P. The importance of patient‐reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014; 35: 2001–2009. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

