Effects of cholesterol on the structure and collapse of DPPC monolayers
- PMID: 35841141
- PMCID: PMC9515002
- DOI: 10.1016/j.bpj.2022.07.007
Effects of cholesterol on the structure and collapse of DPPC monolayers
Abstract
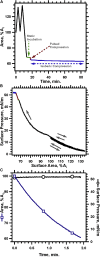
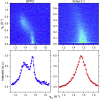
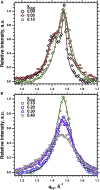
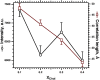
Cholesterol induces faster collapse by compressed films of pulmonary surfactant. Because collapse prevents films from reaching the high surface pressures achieved in the alveolus, most therapeutic surfactants remove or omit cholesterol. The studies here determined the structural changes by which cholesterol causes faster collapse by films of dipalmitoyl phosphatidylcholine, used as a simple model for the functional alveolar film. Measurements of isobaric collapse, with surface pressure held constant at 52 mN/m, showed that cholesterol had little effect until the mol fraction of cholesterol, Xchol, exceeded 0.20. Structural measurements of grazing incidence X-ray diffraction at ambient laboratory temperatures and a surface pressure of 44 mN/m, just below the onset of collapse, showed that the major structural change in an ordered phase occurred at lower Xchol. A centered rectangular unit cell with tilted chains converted to an untilted hexagonal structure over the range of Xchol = 0.0-0.1. For Xchol = 0.1-0.4, the ordered structure was nearly invariant; the hexagonal unit cell persisted, and the spacing of the chains was essentially unchanged. That invariance strongly suggests that above Xchol = 0.1, cholesterol partitions into a disordered phase, which coexists with the ordered domains. The phase rule requires that for a binary film with coexisting phases, the stoichiometries of the ordered and disordered regions must remain constant. Added cholesterol must increase the area of the disordered phase at the expense of the ordered regions. X-ray scattering from dipalmitoyl phosphatidylcholine/cholesterol fit with that prediction. The data also show a progressive decrease in the size of crystalline domains. Our results suggest that cholesterol promotes adsorption not by altering the unit cell of the ordered phase but by decreasing both its total area and the size of individual crystallites.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
Interfacial structure of pulmonary surfactants revisited: Cholesterol and surface pressure effects.Biophys J. 2022 Sep 20;121(18):3305-3306. doi: 10.1016/j.bpj.2022.08.004. Epub 2022 Aug 12. Biophys J. 2022. PMID: 35998615 Free PMC article. No abstract available.
References
-
- Hall S.B., Wang Z., Notter R.H. Separation of subfractions of the hydrophobic components of calf lung surfactant. J. Lipid Res. 1994;35:1386–1394. - PubMed
-
- Postle A.D., Heeley E.L., Wilton D.C. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2001;129:65–73. - PubMed
-
- Bachofen H., Hildebrandt J., Bachofen M. Pressure-volume curves of air- and liquid-filled excised lungs-surface tension in situ. J. Appl. Physiol. 1970;29:422–431. - PubMed
-
- Horie T., Hildebrandt J. Dynamic compliance, limit cycles, and static equilibria of excised cat lung. J. Appl. Physiol. 1971;31:423–430. - PubMed
-
- Valberg P.A., Brain J.D. Lung surface tension and air space dimensions from multiple pressure-volume curves. J. Appl. Physiol. 1977;43:730–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

