Engineered bispecific antibodies targeting the interleukin-6 and -8 receptors potently inhibit cancer cell migration and tumor metastasis
- PMID: 35841152
- PMCID: PMC9637575
- DOI: 10.1016/j.ymthe.2022.07.008
Engineered bispecific antibodies targeting the interleukin-6 and -8 receptors potently inhibit cancer cell migration and tumor metastasis
Abstract
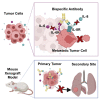
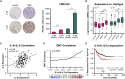
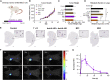
Simultaneous inhibition of interleukin-6 (IL-6) and interleukin-8 (IL-8) signaling diminishes cancer cell migration, and combination therapy has recently been shown to synergistically reduce metastatic burden in a preclinical model of triple-negative breast cancer. Here, we have engineered two novel bispecific antibodies that target the IL-6 and IL-8 receptors to concurrently block the signaling activity of both ligands. We demonstrate that a first-in-class bispecific antibody design has promising therapeutic potential, with enhanced selectivity and potency compared with monoclonal antibody and small-molecule drug combinations in both cellular and animal models of metastatic triple-negative breast cancer. Mechanistic characterization revealed that our engineered bispecific antibodies have no impact on cell viability, but profoundly reduce the migratory potential of cancer cells; hence they constitute a true anti-metastatic treatment. Moreover, we demonstrate that our antibodies can be readily combined with standard-of-care anti-proliferative drugs to develop effective anti-cancer regimens. Collectively, our work establishes an innovative metastasis-focused direction for cancer drug development.
Keywords: antibody; breast cancer; cell migration; interleukins; metastasis.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have filed intellectual property covering the technologies described herein. E.J.M., D.W., and J.B.S. are co-founders of AbMeta Therapeutics.
Figures



Similar articles
-
Mechanistic computational modeling of monospecific and bispecific antibodies targeting interleukin-6/8 receptors.PLoS Comput Biol. 2024 Jun 7;20(6):e1012157. doi: 10.1371/journal.pcbi.1012157. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38848446 Free PMC article.
-
Development of a recombinant anti-VEGFR2-EPCAM bispecific antibody to improve antiangiogenic efficiency.Exp Cell Res. 2021 Aug 15;405(2):112685. doi: 10.1016/j.yexcr.2021.112685. Epub 2021 Jun 6. Exp Cell Res. 2021. PMID: 34090863
-
Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis.Breast Cancer Res. 2018 Jun 14;20(1):54. doi: 10.1186/s13058-018-0981-3. Breast Cancer Res. 2018. PMID: 29898755 Free PMC article.
-
Bispecific Antibodies for Triple Negative Breast Cancer.Trends Cancer. 2021 Feb;7(2):162-173. doi: 10.1016/j.trecan.2020.09.004. Epub 2020 Oct 8. Trends Cancer. 2021. PMID: 33041246 Review.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
Novel bispecific nanobody mitigates experimental intestinal inflammation in mice by targeting TNF-α and IL-23p19 bioactivities.Clin Transl Med. 2024 Mar;14(3):e1636. doi: 10.1002/ctm2.1636. Clin Transl Med. 2024. PMID: 38533646 Free PMC article.
-
Senescence-related genes analysis in breast cancer reveals the immune microenvironment and implications for immunotherapy.Aging (Albany NY). 2024 Feb 14;16(4):3531-3553. doi: 10.18632/aging.205544. Epub 2024 Feb 14. Aging (Albany NY). 2024. PMID: 38358910 Free PMC article.
-
Mechanistic computational modeling of monospecific and bispecific antibodies targeting interleukin-6/8 receptors.PLoS Comput Biol. 2024 Jun 7;20(6):e1012157. doi: 10.1371/journal.pcbi.1012157. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38848446 Free PMC article.
-
Using mathematical modelling and AI to improve delivery and efficacy of therapies in cancer.Nat Rev Cancer. 2025 May;25(5):324-340. doi: 10.1038/s41568-025-00796-w. Epub 2025 Feb 19. Nat Rev Cancer. 2025. PMID: 39972158 Review.
-
The recombinant anti-MET/EpCAM bispecific antibody fragment: a promising novel therapeutic approach for breast cancer treatment.Invest New Drugs. 2025 Jun;43(3):687-700. doi: 10.1007/s10637-025-01546-3. Epub 2025 May 30. Invest New Drugs. 2025. PMID: 40445574
References
-
- Weinberg R.A. W. W. Norton & Company; 2013. The Biology of Cancer.
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. Cancer J. Clin. 2021;71:7–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

