Post-fatigue ability to activate muscle is compromised across a wide range of torques during acute hypoxic exposure
- PMID: 35841186
- PMCID: PMC9546238
- DOI: 10.1111/ejn.15773
Post-fatigue ability to activate muscle is compromised across a wide range of torques during acute hypoxic exposure
Abstract
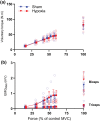
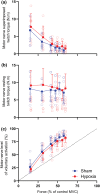
The purpose of this study was to assess how severe acute hypoxia alters the neural mechanisms of muscle activation across a wide range of torque output in a fatigued muscle. Torque and electromyography responses to transcranial and motor nerve stimulation were collected from 10 participants (27 years ± 5 years, 1 female) following repeated performance of a sustained maximal voluntary contraction that reduced torque to 60% of the pre-fatigue peak torque. Contractions were performed after 2 h of hypoxic exposure and during a sham intervention. For hypoxia, peripheral blood oxygen saturation was titrated to 80% over a 15-min period and remained at 80% for 2 h. Maximal voluntary torque, electromyography root mean square, voluntary activation and corticospinal excitability (motor evoked potential area) and inhibition (silent period duration) were then assessed at 100%, 90%, 80%, 70%, 50% and 25% of the target force corresponding to the fatigued maximal voluntary contraction. No hypoxia-related effects were identified for voluntary activation elicited during motor nerve stimulation. However, during measurements elicited at the level of the motor cortex, voluntary activation was reduced at each torque output considered (P = .002, ηp 2 = .829). Hypoxia did not impact the correlative linear relationship between cortical voluntary activation and contraction intensity or the correlative curvilinear relationship between motor nerve voluntary activation and contraction intensity. No other hypoxia-related effects were identified for other neuromuscular variables. Acute severe hypoxia significantly impairs the ability of the motor cortex to voluntarily activate fatigued muscle across a wide range of torque output.
Keywords: corticospinal excitability; exercise; fatigue; hypoxemia; transcranial magnetic stimulation.
© 2022 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
-
- Amann, M. , Blain, G. M. , Proctor, L. T. , Sebranek, J. J. , Pegelow, D. F. , & Dempsey, J. A. (2011). Implications of group III and IV muscle afferents for high‐intensity endurance exercise performance in humans. Journal of Physiology, 589(Pt 21), 5299–5309. 10.1113/jphysiol.2011.213769 - DOI - PMC - PubMed
-
- Amann, M. , Eldridge, M. W. , Lovering, A. T. , Stickland, M. K. , Pegelow, D. F. , & Dempsey, J. A. (2006). Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. Journal of Physiology, 575(Pt 3), 937–952. 10.1113/jphysiol.2006.113936 - DOI - PMC - PubMed
-
- Amann, M. , Goodall, S. , Twomey, R. , Subudhi, A. W. , Lovering, A. T. , & Roach, R. C. (2013). AltitudeOmics: On the consequences of high‐altitude acclimatization for the development of fatigue during locomotor exercise in humans. Journal of Applied Physiology, 115(5), 634–642. 10.1152/japplphysiol.00606.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

