Incidence of Guillain-Barré syndrome following SARS-CoV-2 immunization: Analysis of a nationwide registry of recipients of 81 million doses of seven vaccines
- PMID: 35841212
- PMCID: PMC9349509
- DOI: 10.1111/ene.15504
Incidence of Guillain-Barré syndrome following SARS-CoV-2 immunization: Analysis of a nationwide registry of recipients of 81 million doses of seven vaccines
Abstract
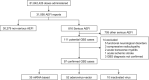
Background and purpose: Information on Guillain-Barré syndrome (GBS) as an adverse event following immunization (AEFI) against SARS-CoV-2 remains scarce. We aimed to report GBS incidence as an AEFI among adult (≥18 years) recipients of 81,842,426 doses of seven anti-SARS-CoV-2 vaccines between December 24, 2020, and October 29, 2021, in Mexico.
Methods: Cases were retrospectively collected through passive epidemiological surveillance. The overall observed incidence was calculated according to the total number of administered doses. Vaccines were analyzed individually and by vector as mRNA-based (mRNA-1273 and BNT162b2), adenovirus-vectored (ChAdOx1 nCov-19, rAd26-rAd5, Ad5-nCoV, and Ad26.COV2-S), and inactivated whole-virion-vectored (CoronaVac) vaccines.
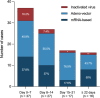
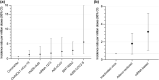
Results: We identified 97 patients (52 males [53.6%]; median [interquartile range] age 44 [33-60] years), for an overall observed incidence of 1.19/1,000,000 doses (95% confidence interval [CI] 0.97-1.45), with incidence higher among Ad26.COV2-S (3.86/1,000,000 doses, 95% CI 1.50-9.93) and BNT162b2 recipients (1.92/1,00,000 doses, 95% CI 1.36-2.71). The interval (interquartile range) from vaccination to GBS symptom onset was 10 (3-17) days. Preceding diarrhea was reported in 21 patients (21.6%) and mild COVID-19 in four more (4.1%). Only 18 patients were tested for Campylobacter jejuni (positive in 16 [88.9%]). Electrophysiological examinations were performed in 76 patients (78.4%; axonal in 46 [60.5%] and demyelinating in 25 [32.8%]); variants were similar across the platforms. On admission, 91.8% had a GBS disability score ≥3. Seventy-five patients (77.3%) received intravenous immunoglobulin, received seven plasma exchange (7.2%), and 15 (15.5%) were treated conservatively. Ten patients (10.3%) died, and 79.1% of survivors were unable to walk independently.
Conclusions: Guillain-Barré syndrome was an extremely infrequent AEFI against SARS-CoV-2. The protection provided by these vaccines outweighs the risk of developing GBS.
Keywords: COVID-19; Guillain-Barré syndrome; SARS-CoV-2; Vaccines; adverse events.
© 2022 European Academy of Neurology.
Figures
Similar articles
-
Exploring the adverse events of Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, and Johnson and Johnson COVID-19 vaccination on Guillain-Barré Syndrome.Sci Rep. 2024 Aug 13;14(1):18767. doi: 10.1038/s41598-024-66999-7. Sci Rep. 2024. PMID: 39138276 Free PMC article.
-
Guillain-Barré syndrome temporally associated with COVID-19 vaccines in Victoria, Australia.Vaccine. 2022 Dec 12;40(52):7579-7585. doi: 10.1016/j.vaccine.2022.10.084. Epub 2022 Nov 7. Vaccine. 2022. PMID: 36357291 Free PMC article.
-
Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States.JAMA Netw Open. 2023 Feb 1;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845. JAMA Netw Open. 2023. PMID: 36723942 Free PMC article.
-
Guillain-Barré syndrome and COVID-19 vaccination: a systematic review and meta-analysis.J Neurol. 2024 Mar;271(3):1063-1071. doi: 10.1007/s00415-024-12186-7. Epub 2024 Jan 17. J Neurol. 2024. PMID: 38233678 Free PMC article.
-
Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature.J Neuroimmunol. 2021 Oct 15;359:577691. doi: 10.1016/j.jneuroim.2021.577691. Epub 2021 Aug 8. J Neuroimmunol. 2021. PMID: 34416410 Free PMC article. Review.
Cited by
-
Validating ICD-10 Diagnosis Codes for Guillain-Barré Syndrome in Taiwan's National Health Insurance Claims Database.Clin Epidemiol. 2024 Oct 21;16:733-742. doi: 10.2147/CLEP.S485953. eCollection 2024. Clin Epidemiol. 2024. PMID: 39445227 Free PMC article.
-
Association Between Guillain-Barré Syndrome and COVID-19 Infection and Vaccination: A Population-Based Nested Case-Control Study.Neurology. 2023 Nov 14;101(20):e2035-e2042. doi: 10.1212/WNL.0000000000207900. Epub 2023 Oct 18. Neurology. 2023. PMID: 37852786 Free PMC article.
-
BCG administration promotes the long-term protection afforded by a single-dose intranasal adenovirus-based SARS-CoV-2 vaccine.iScience. 2023 Aug 11;26(9):107612. doi: 10.1016/j.isci.2023.107612. eCollection 2023 Sep 15. iScience. 2023. PMID: 37670783 Free PMC article.
-
Psychological and Psychiatric Events Following Immunization with Five Different Vaccines against SARS-CoV-2.Vaccines (Basel). 2022 Aug 11;10(8):1297. doi: 10.3390/vaccines10081297. Vaccines (Basel). 2022. PMID: 36016185 Free PMC article.
-
Guillain-Barré syndrome associated with SARS-CoV-2 vaccination: how is it different? a systematic review and individual participant data meta-analysis.Clin Exp Vaccine Res. 2023 Apr;12(2):143-155. doi: 10.7774/cevr.2023.12.2.143. Epub 2023 Apr 30. Clin Exp Vaccine Res. 2023. PMID: 37214140 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

