A hydrogel platform for co-delivery of immunomodulatory proteins for pancreatic islet allografts
- PMID: 35841329
- PMCID: PMC12409600
- DOI: 10.1002/jbm.a.37429
A hydrogel platform for co-delivery of immunomodulatory proteins for pancreatic islet allografts
Abstract
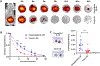
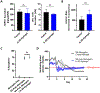
Type 1 diabetes (T1D), an autoimmune disorder in which the insulin-producing β-cells in the islets of Langerhans in the pancreas are destroyed, afflicts over 1.6 million Americans. Although pancreatic islet transplantation has shown promise in treating T1D, continuous use of required immunosuppression regimens limits clinical islet transplantation as it poses significant adverse effects on graft recipients and does not achieve consistent long-term graft survival with 50%-70% of recipients maintaining insulin independence at 5 years. T cells play a key role in graft rejection, and rebalancing pathogenic T effector and protective T regulatory cells can regulate autoimmune disorders and transplant rejection. The synergy of the interleukin-2 (IL-2) and Fas immunomodulatory pathways presents an avenue for eliminating the need for systemic immune suppression by exploiting IL-2's role in expanding regulatory T cells and leveraging Fas ligand (FasL) activity on antigen-induced cell death of effector T cells. Herein, we developed a hydrogel platform for co-delivering an analog of IL-2, IL-2D, and FasL-presenting microgels to achieve localized immunotolerance to pancreatic islets by targeting the upregulation of regulatory T cells and effector T cells simultaneously. Although this hydrogel provided for sustained, local delivery of active immunomodulatory proteins, indefinite allograft survival was not achieved. Immune profiling analysis revealed upregulation of target regulatory T cells but also increases in Granzyme B-expressing CD8+ T cells at the graft site. We attribute the failed establishment of allograft survival to these Granzyme B-expressing T cells. This study underscores the delicate balance of immunomodulatory components important for allograft survival - whose outcome can be dependent on timing, duration, modality of delivery, and disease model.
Keywords: cell transplantation; immunomodulation; type 1 diabetes.
© 2022 Wiley Periodicals LLC.
Figures
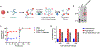




References
-
- Federation, I.D IDF Diabetes Atlas. 9th edition (2019).
-
- Katsarou Anastasia, S. G, Rawshani Araz, Dabelea Dana Type 1 diabetes mellitus. Nature Reviews (2017). - PubMed
-
- Shapiro AM, Pokrywczynska M & Ricordi C Clinical pancreatic islet transplantation. Nature reviews. Endocrinology 13, 268–277 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

