Pattern of Clinical Progression Until Metastatic Castration-Resistant Prostate Cancer: An Epidemiological Study from the European Prostate Cancer Registry
- PMID: 35841526
- PMCID: PMC9345837
- DOI: 10.1007/s11523-022-00899-6
Pattern of Clinical Progression Until Metastatic Castration-Resistant Prostate Cancer: An Epidemiological Study from the European Prostate Cancer Registry
Abstract
Background: Prostate cancer (PCa) is the most frequently diagnosed cancer in men in Europe. The impact of PCa natural history and therapeutic management on the outcomes of castration-resistant prostate cancer patients with metastasis (mCRPC) remains unclear.
Objective: The objective of this study was to describe retrospectively patterns of clinical progression through diagnosis sequences before the mCRPC stage and to assess how these sequences impacted patients' disease progression and overall survival at mCRPC stage.
Patients and methods: Patients with mCRPC were identified from the Prostate Cancer Registry (PCR), an observational study in a real-world setting in 16 countries between 2013 and 2016. Patients were grouped in diagnosis sequences before mCRPC and defined by date of PCa diagnosis, first metastasis, and castration resistance. Distribution of time-to-event variables were estimated using Kaplan-Meier product-limit survival curves for overall survival (OS) and progression-free survival (PFS). Non-adjusted Cox models were conducted for efficacy endpoints (OS, PFS) to estimate hazard ratios between diagnosis sequences.
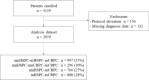
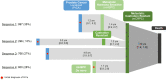
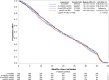
Results: At the end of study, 2859 mCRPC patients were included in this analysis. Among mCRPC four diagnosis sequences were identified: 35% developed metastases (mHSPC) before becoming castration resistant (sequence 1, metachronous mHSPC), 10% developed castration resistance (nmCRPC) before metastases (sequence 2), 27% developed metastases and castration resistance within 4 months (sequence 3) and 28% of patients were de novo mHSPC (sequence 4). Median OS was 17.7 months (interquartile range (IQR): 8.8-29.9) and PFS was 6.4 months (IQR: 3.2-12.0). The univariate analyses showed no correlation between mCRPC patients' OS or PFS and the diagnosis sequence.
Conclusion: This large European study describe four different patterns of prostate cancer progression to mCRPC stage. Our results indicate that patient survival becomes comparable after progression to mCRPC, regardless of the diagnosis sequence.
Trial registration: ClinicalTrials.gov identifier NCT02236637; registered September 2014.
© 2022. The Author(s).
Conflict of interest statement
Camille Verry, Sébastien Vincendeau, and Marc-Olivier Timsit received fees from Janssen-Cilag to participate in the conception, design, interpretations, and the writing of these secondary analyses presented in this manuscript. Marie-Laure Bazil, Pauline Bernardini, and Ségolène Pettré are employees of Janssen-Cilag, Issy les Moulineaux, France. Marc Massetti, Martin Blachier, and Alexandre Vimont are employees of Public Health Expertise which was contracted by Janssen-Cilag, France to perform the Prostate Cancer Registry (PCR) secondary analyses and participate in the writing of the manuscript.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

