Absent or suboptimal response to booster dose of COVID-19 vaccine in patients with autoimmune systemic diseases
- PMID: 35841684
- PMCID: PMC9271490
- DOI: 10.1016/j.jaut.2022.102866
Absent or suboptimal response to booster dose of COVID-19 vaccine in patients with autoimmune systemic diseases
Abstract
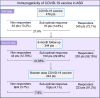
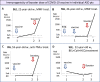
Autoimmune systemic diseases (ASD) show impaired immunogenicity to COVID-19 vaccines. Our prospective observational multicenter study aimed at evaluating the seroconversion elicited by COVID-19 vaccine over the entire vaccination cycle including the booster dose. Among 478 unselected ASD patients originally evaluated at the end of the first vaccination cycle (time 1), 344 individuals were re-evaluated after a 6-month period (time 2), and 244 after the booster vaccine dose (time 3). The immunogenicity of mRNA COVID-19 vaccines (BNT162b2 and mRNA-1273) was assessed by measuring serum IgG-neutralizing antibody (NAb) on samples obtained at the three time points in both patients and 502 age-matched controls. In the 244 ASD group that received booster vaccine and monitored over the entire follow-up, the mean serum NAb levels (time 1, 2, and 3: 696.8 ± 52.68, 370.8 ± 41.92, and 1527 ± 74.16SD BAU/mL, respectively; p < 0.0001) were constantly lower compared to controls (p < 0.0001), but they significantly increased after the booster dose compared to the first two measurements (p < 0.0001). The percentage of patients with absent/suboptimal response to vaccine significantly decreased after the booster dose compared to the first and second evaluations (time 1, 2, and 3: from 28.2% to 46.3%, and to 7.8%, respectively; p < 0.0001). Of note, the percentage of patients with absent/suboptimal response after the booster dose was significantly higher compared to controls (19/244, 7.8% vs 1/502, 0.2%; p < 0.0001). Similarly, treatment with immune-modifiers increased the percentage of patients exhibiting absent/suboptimal response (16/122, 13.1% vs 3/122, 2.46%; p = 0.0031). Overall, the above findings indicate the usefulness of booster vaccine administration in ASD patients. Moreover, the persistence of a significantly higher percentage of individuals without effective seroconversion (7.8%), even after the booster dose, warrants for careful monitoring of NAb levels in all ASD patients to identify those with increased risk of infection. In this particularly frail patients' setting, tailored vaccination and/or therapeutic strategy are highly advisable.
Keywords: Autoimmune systemic diseases; Booster vaccine; COVID-19 vaccine; Cryoglobulinemic vasculitis; Neutralizing antibodies; Rheumatoid arthritis; Systemic lupus; Systemic sclerosis; Systemic vasculitis.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures

References
-
- Pablos J.L., Galindo M., Carmona L., Lledó A., Retuerto M., Blanco R., et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann. Rheum. Dis. 2020;79:1544–1549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

