The airway smooth muscle sodium/calcium exchanger NCLX is critical for airway remodeling and hyperresponsiveness in asthma
- PMID: 35841929
- PMCID: PMC9372629
- DOI: 10.1016/j.jbc.2022.102259
The airway smooth muscle sodium/calcium exchanger NCLX is critical for airway remodeling and hyperresponsiveness in asthma
Abstract
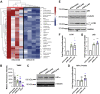
The structural changes of airway smooth muscle (ASM) that characterize airway remodeling (AR) are crucial to the pathogenesis of asthma. During AR, ASM cells dedifferentiate from a quiescent to a proliferative, migratory, and secretory phenotype. Calcium (Ca2+) is a ubiquitous second messenger that regulates many cellular processes, including proliferation, migration, contraction, and metabolism. Furthermore, mitochondria have emerged as major Ca2+ signaling organelles that buffer Ca2+ through uptake by the mitochondrial Ca2+ uniporter and extrude it through the Na+/Ca2+ exchanger (NCLX/Slc8b1). Here, we show using mitochondrial Ca2+-sensitive dyes that NCLX only partially contributes to mitochondrial Ca2+ extrusion in ASM cells. Yet, NCLX is necessary for ASM cell proliferation and migration. Through cellular imaging, RNA-Seq, and biochemical assays, we demonstrate that NCLX regulates these processes by preventing mitochondrial Ca2+ overload and supporting store-operated Ca2+ entry, activation of Ca2+/calmodulin-dependent kinase II, and transcriptional and metabolic reprogramming. Using small animal respiratory mechanic measurements and immunohistochemistry, we show that smooth muscle-specific NCLX KO mice are protected against AR, fibrosis, and hyperresponsiveness in an experimental model of asthma. Our findings support NCLX as a potential therapeutic target in the treatment of asthma.
Keywords: CaMKII; NCLX; SOCE; airway fibrosis; airway hyperresponsiveness; airway remodeling; asthma; calcium signaling; metabolism; mitochondrial calcium.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann. Am. Thorac. Soc. 2018;15:348–356. - PubMed
-
- Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43:343–373. - PubMed
-
- Bourdin A., Neveu D., Vachier I., Paganin F., Godard P., Chanez P. Specificity of basement membrane thickening in severe asthma. J. Allergy Clin. Immunol. 2007;119:1367–1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

