Cell-free DNA screening positive for monosomy X: clinical evaluation and management of suspected maternal or fetal Turner syndrome
- PMID: 35841934
- PMCID: PMC9729468
- DOI: 10.1016/j.ajog.2022.07.004
Cell-free DNA screening positive for monosomy X: clinical evaluation and management of suspected maternal or fetal Turner syndrome
Abstract
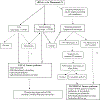
Initially provided as an alternative to evaluation of serum analytes and nuchal translucency for the assessment of pregnancies at high risk of trisomy 21, cell-free DNA screening for fetal aneuploidy, also referred to as noninvasive prenatal screening, can now also screen for fetal sex chromosome anomalies such as monosomy X as early as 9 to 10 weeks of gestation. Early identification of Turner syndrome, a sex chromosome anomaly resulting from the complete or partial absence of the second X chromosome, allows medical interventions such as optimizing obstetrical outcomes, hormone replacement therapy, fertility preservation and support, and improved neurocognitive outcomes. However, cell-free DNA screening for sex chromosome anomalies and monosomy X in particular is associated with high false-positive rates and low positive predictive value. A cell-free DNA result positive for monosomy X may represent fetal Turner syndrome, maternal Turner syndrome, or confined placental mosaicism. A positive screen for monosomy X with discordant results of diagnostic fetal karyotype presents unique interpretation and management challenges because of potential implications for previously unrecognized maternal Turner syndrome. The current international consensus clinical practice guidelines for the care of individuals with Turner syndrome throughout the lifespan do not specifically address management of individuals with a cell-free DNA screen positive for monosomy X. This study aimed to provide context and expert-driven recommendations for maternal and/or fetal evaluation and management when cell-free DNA screening is positive for monosomy X. We highlight unique challenges of cell-free DNA screening that is incidentally positive for monosomy X, present recommendations for determining if the result is a true-positive, and discuss when diagnosis of Turner syndrome is applicable to the fetus vs the mother. Whereas we defer the subsequent management of confirmed Turner syndrome to the clinical practice guidelines, we highlight unique considerations for individuals initially identified through cell-free DNA screening.
Keywords: Turner syndrome; cell-free DNA; monosomy X; noninvasive prenatal testing; sex-chromosome anomalies.
Published by Elsevier Inc.
Conflict of interest statement
Figures



References
-
- Nicolaides KH, et al., Assessment of fetal sex chromosome aneuploidy using directed cell-free DNA analysis. Fetal Diagn Ther, 2014. 35(1): p. 1–6. - PubMed
-
- Bianchi DW, et al., Massively parallel sequencing of maternal plasma DNA in 113 cases of fetal nuchal cystic hygroma. Obstet Gynecol, 2013. 121(5): p. 1057–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

