Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia
- PMID: 35842477
- PMCID: PMC9288483
- DOI: 10.1038/s41598-022-16476-w
Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia
Abstract
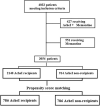
We evaluated the effect of Acetyl-cholinesterase-inhibitors (AChEIs) on cognitive decline and overall survival in a large sample of older patients with late onset Alzheimer's disease (LOAD), vascular dementia (VD) or Lewy body disease (LBD) from a real world setting. Patients with dementia enrolled between 2005 and 2020 by the "Alzheimer's Disease Research Centers" were analysed; the mean follow-up period was 7.9 years. A 1:1 propensity score matching was performed generating a cohort of 1.572 patients (786 treated [AChEIs +] and 786 not treated [AChEIs-] with AChEIs. The MMSE score was almost stable during the first 6 years of follow up in AChEIs + and then declined, while in AChEIs- it progressively declined so that at the end of follow-up (13.6 years) the average decrease in MMSE was 10.8 points in AChEIs- compared with 5.4 points in AChEIs + (p < 0.001). This trend was driven by LOAD (Δ-MMSE:-10.8 vs. -5.7 points; p < 0.001), although a similar effect was observed in VD (Δ-MMSE:-11.6 vs. -8.8; p < 0.001). No effect on cognitive status was found in LBD. At multivariate Cox regression analysis (adjusted for age, gender, dependency level and depression) a strong association between AChEIs therapy and lower all-cause mortality was observed (H.R.:0.59; 95%CI: 0.53-0.66); this was confirmed also in analyses separately conducted in LOAD, VD and LBD. Among older people with dementia, treatment with AChEIs was associated with a slower cognitive decline and with reduced mortality, after a mean follow-up of almost eight years. Our data support the effectiveness of AChEIs in older patients affected by these types of dementia.
© 2022. The Author(s).
Conflict of interest statement
AC reports personal fees from Nestle, personal fees from Bristol Myers Squibb and payment /honoraria from MSD, outside the submitted work; SV reports Consulting fees from Angelini SPA and Payment/honoraria from Abbot Vifor, outside from the submitted work. MZ, GZ and LF have no conflicts of interest to disclose.
Figures


Similar articles
-
Memantine and Acetylcholinesterase Inhibitor Use in Alzheimer's Disease Clinical Trials: Potential for Confounding by Indication.J Alzheimers Dis. 2019;67(2):707-713. doi: 10.3233/JAD-180684. J Alzheimers Dis. 2019. PMID: 30636733
-
Greater Variability in Cognitive Decline in Lewy Body Dementia Compared to Alzheimer's Disease.J Alzheimers Dis. 2020;73(4):1321-1330. doi: 10.3233/JAD-190731. J Alzheimers Dis. 2020. PMID: 31903991
-
Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality.Neurology. 2021 Apr 27;96(17):e2220-e2230. doi: 10.1212/WNL.0000000000011832. Epub 2021 Mar 19. Neurology. 2021. PMID: 33741639 Free PMC article.
-
Acetyl-cholinesterase-inhibitors reconsidered. A narrative review of post-marketing studies on Alzheimer's disease.Aging Clin Exp Res. 2024 Feb 7;36(1):23. doi: 10.1007/s40520-023-02675-6. Aging Clin Exp Res. 2024. PMID: 38321321 Free PMC article. Review.
-
Effect of Apolipoprotein E ɛ4 Carrier Status on Cognitive Response to Acetylcholinesterase Inhibitors in Patients with Alzheimer's Disease: A Systematic Review and Meta-Analysis.Dement Geriatr Cogn Disord. 2018;45(5-6):335-352. doi: 10.1159/000490175. Epub 2018 Jul 24. Dement Geriatr Cogn Disord. 2018. PMID: 30041236
Cited by
-
Alterations in cognitive function and blood biomarkers following transcranial direct current stimulation in patients with amyloid positron emission tomography-positive Alzheimer's disease: a preliminary study.Front Neurosci. 2023 Dec 21;17:1327886. doi: 10.3389/fnins.2023.1327886. eCollection 2023. Front Neurosci. 2023. PMID: 38178837 Free PMC article.
-
Evaluation of [125I]α-Bungarotoxin Binding to α7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Alzheimer's Disease Brain.Receptors (Basel). 2025 Mar;4(1):7. doi: 10.3390/receptors4010007. Epub 2025 Mar 20. Receptors (Basel). 2025. PMID: 40678024 Free PMC article.
-
A how-to guide for a precision medicine approach to the diagnosis and treatment of Alzheimer's disease.Front Aging Neurosci. 2023 Aug 17;15:1213968. doi: 10.3389/fnagi.2023.1213968. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37662550 Free PMC article. Review.
-
Design, synthesis, and pharmacological evaluation of heteroaryl thiol-linked kojic acid derivatives as a novel class of acetylcholinesterase inhibitors for Alzheimer's disease therapy.3 Biotech. 2025 May;15(5):134. doi: 10.1007/s13205-025-04295-5. Epub 2025 Apr 18. 3 Biotech. 2025. PMID: 40255452
-
New 1,2,4-oxadiazole derivatives as potential multifunctional agents for the treatment of Alzheimer's disease: design, synthesis, and biological evaluation.BMC Chem. 2024 Jul 13;18(1):130. doi: 10.1186/s13065-024-01235-x. BMC Chem. 2024. PMID: 39003489 Free PMC article.
References
-
- Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, Green C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int. J. Geriatr. Psychiatry. 2006;21:17–28. doi: 10.1002/gps.1402. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG016574/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG033514/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- K23 AG031861/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States

