Targeting IL-6 by engineered Lactococcus lactis via surface-displayed affibody
- PMID: 35842694
- PMCID: PMC9287920
- DOI: 10.1186/s12934-022-01873-7
Targeting IL-6 by engineered Lactococcus lactis via surface-displayed affibody
Abstract
Background: Dysregulated production of interleukin (IL)-6 is implicated in the pathology of inflammatory bowel disease (IBD). Neutralization of IL-6 in the gut by safe probiotic bacteria may help alleviate intestinal inflammation. Here, we developed Lactococcus lactis with potent and selective IL-6 binding activity by displaying IL-6-specific affibody on its surface.
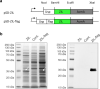
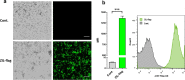
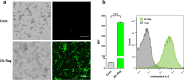
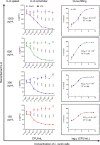
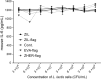
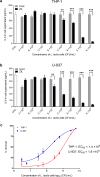
Results: Anti-IL-6 affibody (designated as ZIL) was expressed in fusion with lactococcal secretion peptide Usp45 and anchoring protein AcmA. A high amount of ZIL fusion protein was detected on bacterial surface, and its functionality was validated by confocal microscopy and flow cytometry. Removal of IL-6 from the surrounding medium by the engineered L. lactis was evaluated using enzyme-linked immunosorbent assay. ZIL-displaying L. lactis sequestered recombinant human IL-6 from the solution in a concentration-dependent manner by up to 99% and showed no binding to other pro-inflammatory cytokines, thus proving to be highly specific for IL-6. The removal was equally efficient across different IL-6 concentrations (150-1200 pg/mL) that were found to be clinically relevant in IBD patients. The ability of engineered bacteria to capture IL-6 from cell culture supernatant was assessed using immunostimulated human monocytic cell lines (THP-1 and U-937) differentiated into macrophage-like cells. ZIL-displaying L. lactis reduced the content of IL-6 in the supernatants of both cell lines in a concentration-dependent manner by up to 94%. Dose response analysis showed that bacterial cell concentrations of 107 and 109 CFU/mL (colony forming units per mL) were required for half-maximal removal of recombinant and macrophage-derived IL-6, respectively.
Conclusion: The ability of ZIL-displaying L. lactis to bind pathological concentrations of IL-6 at common bacterial doses suggests physiological significance.
Keywords: Delivery system; IL-6; Inflammatory bowel disease; Lactococcus lactis; Microbiota.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
p19-Targeting ILP Protein Blockers of IL-23/Th-17 Pro-Inflammatory Axis Displayed on Engineered Bacteria of Food Origin.Int J Mol Sci. 2018 Jul 1;19(7):1933. doi: 10.3390/ijms19071933. Int J Mol Sci. 2018. PMID: 29966384 Free PMC article.
-
Engineered and wild-type L. lactis promote anti-inflammatory cytokine signalling in inflammatory bowel disease patient's mucosa.World J Microbiol Biotechnol. 2019 Feb 27;35(3):45. doi: 10.1007/s11274-019-2615-z. World J Microbiol Biotechnol. 2019. PMID: 30810891
-
Lactic Acid Bacteria with Concomitant IL-17, IL-23 and TNFα- Binding Ability for the Treatment of Inflammatory Bowel Disease.Curr Pharm Biotechnol. 2017;18(4):318-326. doi: 10.2174/1389201018666170210152218. Curr Pharm Biotechnol. 2017. PMID: 28190384
-
In situ delivery of cytokines by genetically engineered Lactococcus lactis.Antonie Van Leeuwenhoek. 2002 Aug;82(1-4):323-31. Antonie Van Leeuwenhoek. 2002. PMID: 12369199 Review.
-
Lactococcus lactis as a cell factory for delivery of therapeutic proteins.Curr Gene Ther. 2010 Feb;10(1):34-45. doi: 10.2174/156652310790945557. Curr Gene Ther. 2010. PMID: 20156189 Review.
Cited by
-
Recent advances in bacteria-based platforms for inflammatory bowel diseases treatment.Exploration (Beijing). 2024 Mar 5;4(5):20230142. doi: 10.1002/EXP.20230142. eCollection 2024 Oct. Exploration (Beijing). 2024. PMID: 39439496 Free PMC article. Review.
-
Engineered Probiotic-Based Biomaterials for Inflammatory Bowel Disease Treatment.Theranostics. 2025 Feb 18;15(8):3289-3315. doi: 10.7150/thno.103983. eCollection 2025. Theranostics. 2025. PMID: 40093907 Free PMC article. Review.
-
Genetically Engineered Microorganisms and Their Impact on Human Health.Int J Clin Pract. 2024 Mar 9;2024:6638269. doi: 10.1155/2024/6638269. eCollection 2024. Int J Clin Pract. 2024. PMID: 38495751 Free PMC article. Review.
-
Incorporation of recombinant proteins into extracellular vesicles by Lactococcus cremoris.Sci Rep. 2025 Jan 13;15(1):1768. doi: 10.1038/s41598-025-86492-z. Sci Rep. 2025. PMID: 39815011 Free PMC article.
References
-
- Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. - DOI - PMC - PubMed
-
- Reinisch W, Hommes DW, Van Assche G, Colombel JF, Gendre JP, Oldenburg B, et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn's disease. Gut. 2006;55:1138–1144. doi: 10.1136/gut.2005.079434. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

