Long-term survival of patients with central or > 7 cm T4 N0/1 M0 non-small-cell lung cancer treated with definitive concurrent radiochemotherapy in comparison to trimodality treatment
- PMID: 35842712
- PMCID: PMC9288731
- DOI: 10.1186/s13014-022-02080-9
Long-term survival of patients with central or > 7 cm T4 N0/1 M0 non-small-cell lung cancer treated with definitive concurrent radiochemotherapy in comparison to trimodality treatment
Abstract
Abstarct: BACKGROUND: To examine long-term-survival of cT4 cN0/1 cM0 non-small-cell lung carcinoma (NSCLC) patients undergoing definitive radiochemotherapy (ccRTx/CTx) in comparison to the trimodality treatment, neoadjuvant radiochemotherapy followed by surgery, at a high volume lung cancer center.
Methods: All consecutive patients with histopathologically confirmed NSCLC (cT4 cN0/1 cM0) with a curative-intent-to-treat ccRTx/CTx were included between 01.01.2001 and 01.07.2019. Mediastinal involvement was excluded by systematic EBUS-TBNA or mediastinoscopy. Following updated T4-stage-defining-criteria initial staging was reassessed by an expert-radiologist according to UICC-guidelines [8th edition]. Outcomes were compared with previously reported results from patients of the same institution with identical inclusion criteria, who had been treated with neoadjuvant radiochemotherapy and resection. Factors for treatment selection were documented. Endpoints were overall-survival (OS), progression-free-survival (PFS), and cumulative incidences of isolated loco-regional failures, distant metastases, secondary tumors as well as non-cancer deaths within the first year.
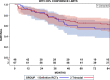
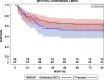
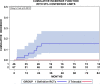
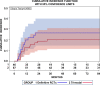
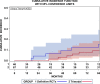
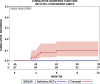
Results: Altogether 46 consecutive patients with histopathologically confirmed NSCLC cT4 cN0/1 cM0 [cN0 in 34 and cN1 in 12 cases] underwent ccRTx/CTx after induction chemotherapy (iCTx). Median follow-up was 133 months. OS-rates at 3-, 5-, and 7-years were 74.9%, 57.4%, and 57.4%, respectively. Absolute OS-rate of ccRTx/CTx at 5 years were within 10% of the trimodality treatment reference group (Log-Rank p = 0.184). The cumulative incidence of loco-regional relapse was higher after iCTx + ccRT/CTx (15.2% vs. 0% at 3 years, p = 0.0012, Gray's test) while non-cancer deaths in the first year were lower than in the trimodality reference group (0% vs 9.1%, p = 0.0360, Gray's test). None of the multiple recorded prognostic parameters were significantly associated with survival after iCTx + ccRT/CTx: Propensity score weighting for adjustment of prognostic factors between iCTx + ccRT/CTx and trimodality treatment did not change the results of the comparisons.
Conclusions: Patients with cT4 N0/1 M0 NSCLC have comparable OS with ccRTx/CTx and trimodality treatment. Loco-regional relapses were higher and non-cancer related deaths lower with ccRTx/CTx. Definitive radiochemotherapy is an adequate alternative for patients with an increased risk of surgery-related morbidity.
Keywords: Definitive radiochemotherapy; Non-small cell lung cancer; TNM-staging.
© 2022. The Author(s).
Conflict of interest statement
PD Dr. med. N Guberina, PD Dr. med. M Guberina, Dr. med. M Metzenmacher, Prof. Dr. med. K Darwiche, PD Dr. med. T Ploenes, Prof. Dr. med. M Schuler, Prof. Dr. med. D Theegarten, Prof.. Dr. med. G. Stamatis, Prof. Dr. med. C Aigner: There are no relationships/conditions/circumstances that present a potential conflict of interest. All authors declare that they have no conflict of interest. Prof. Dr. med. C Pöttgen reports personal fees from Roche Pharma, personal fees from Boehringer Ingelheim, personal fees from Astra Zeneca, all outside the submitted work; .There are no relationships/conditions/circumstances that present a potential conflict of interest. PD Dr. med. W:E:E. Eberhardt reports honoraria (Advisory board function) from Astra Zeneca, BMS, Roche, MSD, Pfizer, Boehringer, Takeda, Eli Lilly, Bayer, Celgene Honoraria, and (educational lectures) from BMS, MSD Astra Zeneca, Roche, Novartis, Pfizer, Boehringer, Takeda, Abbvie, Celgene, Eli Lilly as well as (research grants) from Eli Lilly, BMS, and Astra Zeneca. The author declares that no competing interests exist. Dr med. T. Gauler reports advisory board/consultant function for Ipsen, No-vartis, BMS, Eisai and honoraria from BMS, Ipsen, Novartis, MSD, Eisai, Pfizer as well as traveling expenses from BMS, Ipsen, Novartis, MSD, Eisai, Pfizer and stocks from Bayer. Prof. Dr. med. M. Stuschke reports research grants contributed by AstraZeneca in 2019 and 2020. Professor Dr.med. M. Stuschke confirms that the above mentioned funding source was not involved in the study design or materials used, nor in the collection, analysis, and interpretation of data nor in the writing of the paper.
Figures
Similar articles
-
Comparison of early tumour-associated versus late deaths in patients with central or >7 cm T4 N0/1 M0 non-small-cell lung-cancer undergoing trimodal treatment: Only few risks left to improve.Eur J Cancer. 2020 Oct;138:156-168. doi: 10.1016/j.ejca.2020.07.025. Epub 2020 Sep 2. Eur J Cancer. 2020. PMID: 32889370
-
Recurrence dynamics after trimodality therapy (Neoadjuvant concurrent chemoradiotherapy and surgery) in patients with stage IIIA (N2) lung cancer.Lung Cancer. 2018 Jan;115:89-96. doi: 10.1016/j.lungcan.2017.11.020. Epub 2017 Nov 22. Lung Cancer. 2018. PMID: 29290268
-
Impact of lung function changes after induction radiochemotherapy on resected T4 non-small cell lung cancer outcome.Ann Thorac Surg. 2012 Dec;94(6):1815-22. doi: 10.1016/j.athoracsur.2012.08.054. Epub 2012 Oct 25. Ann Thorac Surg. 2012. PMID: 23103000
-
Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC) - a cumulative meta-analysis of the randomized evidence.Oncotarget. 2017 Jun 20;8(25):41670-41678. doi: 10.18632/oncotarget.16471. Oncotarget. 2017. PMID: 28415831 Free PMC article. Review.
-
The present status of surgery for lung cancer.Acta Chir Belg. 1996 Nov-Dec;96(6):245-51. Acta Chir Belg. 1996. PMID: 9008764 Review.
References
-
- Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e369S–e399S. doi: 10.1378/chest.12-2362. - DOI - PubMed
-
- Goldstraw P, Chansky K, Crowley J, et al. International association for the study of lung cancer staging and prognostic factors committee, advisory boards, and participating institutions; international association for the study of lung cancer staging and prognostic factors committee advisory boards and participating institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

