Notch-activated mesenchymal stromal/stem cells enhance the protective effect against acetaminophen-induced acute liver injury by activating AMPK/SIRT1 pathway
- PMID: 35842731
- PMCID: PMC9288678
- DOI: 10.1186/s13287-022-02999-6
Notch-activated mesenchymal stromal/stem cells enhance the protective effect against acetaminophen-induced acute liver injury by activating AMPK/SIRT1 pathway
Abstract
Background: Notch signaling plays important roles in regulating innate immunity. However, little is known about the role of Notch in mesenchymal stromal/stem cell (MSC)-mediated immunomodulation during liver inflammatory response.
Methods: Notch activation in human umbilical cord-derived MSCs was performed by a tissue culture plate coated with Notch ligand, recombinant human Jagged1 (JAG1). Mice were given intravenous injection of Notch-activated MSCs after acetaminophen (APAP)-induced acute liver injury. Liver tissues were collected and analyzed by histology and immunohistochemistry.
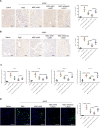
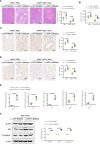
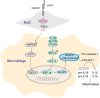
Results: MSC administration reduced APAP-induced hepatocellular damage, as manifested by decreased serum ALT levels, intrahepatic macrophage/neutrophil infiltration, hepatocellular apoptosis and proinflammatory mediators. The anti-inflammatory activity and therapeutic effects of MSCs were greatly enhanced by Notch activation via its ligand JAG1. However, Notch2 disruption in MSCs markedly diminished the protective effect of MSCs against APAP-induced acute liver injury, even in the presence of JAG1 pretreatment. Strikingly, Notch-activated MSCs promoted AMP-activated protein kinase (AMPKα) phosphorylation, increased the sirtuins 1 (SIRT1) deacetylase expression, but downregulated spliced X-box-binding protein 1 (XBP1s) expression and consequently reduced NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation. Furthermore, SIRT1 disruption or XBP1s overexpression in macrophages exacerbated APAP-triggered liver inflammation and augmented NLRP3/caspase-1 activity in MSC-administrated mice. Mechanistic studies further demonstrated that JAG1-pretreated MSCs activated Notch2/COX2/PGE2 signaling, which in turn induced macrophage AMPK/SIRT1 activation, leading to XBP1s deacetylation and inhibition of NLRP3 activity.
Conclusions: Activation of Notch2 is required for the ability of MSCs to reduce the severity of APAP-induced liver damage in mice. Our findings underscore a novel molecular insights into MSCs-mediated immunomodulation by activating Notch2/COX2/AMPK/SIRT1 pathway and thus provide a new strategy for the treatment of liver inflammatory diseases.
Keywords: Acute liver injury; Mesenchymal stromal/stem cell; Notch signaling; SIRT1; XBP1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Biagioli M, Carino A, Fiorucci C, Marchiano S, Di Giorgio C, Bordoni M, et al. The bile acid receptor GPBAR1 modulates CCL2/CCR2 signaling at the liver sinusoidal/macrophage interface and reverses acetaminophen-induced liver toxicity. J Immunol. 2020;204:2535–2551. doi: 10.4049/jimmunol.1901427. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

