Mitochondrial DNA mutations in ageing and cancer
- PMID: 35842901
- PMCID: PMC9490137
- DOI: 10.1002/1878-0261.13291
Mitochondrial DNA mutations in ageing and cancer
Abstract
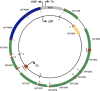
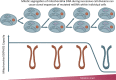
Advancing age is a major risk factor for malignant transformation and the development of cancer. As such, over 50% of neoplasms occur in individuals over the age of 70. The pathologies of both ageing and cancer have been characterized by respective groups of molecular hallmarks, and while some features are divergent between the two pathologies, several are shared. Perturbed mitochondrial function is one such common hallmark, and this observation therefore suggests that mitochondrial alterations may be of significance in age-related cancer development. There is now considerable evidence documenting the accumulation of somatic mitochondrial DNA (mtDNA) mutations in ageing human postmitotic and replicative tissues. Similarly, mutations of the mitochondrial genome have been reported in human cancers for decades. The plethora of functions in which mitochondria partake, such as oxidative phosphorylation, redox balance, apoptosis and numerous biosynthetic pathways, manifests a variety of ways in which alterations in mtDNA may contribute to tumour growth. However, the specific mechanisms by which mtDNA mutations contribute to tumour progression remain elusive and often contradictory. This review aims to consolidate current knowledge and describe future direction within the field.
Keywords: ageing; cancer; metabolism; mitochondria; mitochondrial DNA; oxidative phosphorylation.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Belitzer V, Tsybakova E. The mechanism of phosphorylation associated with respiration. Biochimiya. 1939;4:516–34.
-
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70(2):391–425. - PubMed
-
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell‐free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–57. - PubMed
-
- Sano S, Inoue S, Tanabe Y, Sumiya C, Koike S. Significance of mitochondria for porphyrin and heme biosynthesis. Science (New York, NY). 1959;129(3344):275–6. - PubMed
-
- Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

