Interactions of the protease inhibitor, ritonavir, with common anesthesia drugs
- PMID: 35842922
- PMCID: PMC9543968
- DOI: 10.1111/pan.14529
Interactions of the protease inhibitor, ritonavir, with common anesthesia drugs
Abstract
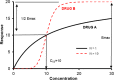
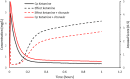
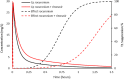
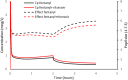
The protease inhibitor, ritonavir, is a strong inhibitor of CYP 3A. The drug is used for management of the human immunovirus and is currently part of an oral antiviral drug combination (nirmatrelvir-ritonavir) for the early treatment of SARS-2 COVID-19-positive patients aged 12 years and over who have recognized comorbidities. The CYP 3A enzyme system is responsible for clearance of numerous drugs used in anesthesia (e.g., alfentanil, fentanyl, methadone, rocuronium, bupivacaine, midazolam, ketamine). Ritonavir will have an impact on drug clearances that are dependent on ritonavir concentration, anesthesia drug intrinsic hepatic clearance, metabolic pathways, concentration-response relationship, and route of administration. Drugs with a steep concentration-response relationship (ketamine, midazolam, rocuronium) are mostly affected because small changes in concentration have major changes in effect response. An increase in midazolam concentration is observed after oral administration because CYP 3A in the gastrointestinal wall is inhibited, causing a large increase in relative bioavailability. Fentanyl infusion may be associated with a modest increase in plasma concentration and effect, but the large between subject variability of pharmacokinetic and pharmacodynamic concentration changes suggests it will have little impact on an individual patient, especially when used with adverse effect monitoring. It has been proposed that drugs that have no or only a small metabolic pathway involving the CYP 3A enzyme be used during anesthesia, for example, propofol, atracurium, remifentanil, and the volatile agents. That anesthesia approach denies children of drugs with considerable value. It is better that the inhibitory changes in clearance of these drugs are understood so that rational drug choices can be made to tailor drug use to the individual patient. Altered drug dose, anticipation of duration of effect, timing of administration, use of reversal agents and perioperative monitoring would better behoove children undergoing anesthesia.
Keywords: COVID-19; anesthesia; antiviral; children; drug interactions; pharmacodynamics.
© 2022 The Authors. Pediatric Anesthesia published by John Wiley & Sons Ltd.
Conflict of interest statement
AS and HH have no conflicts of interest to declare. BJA is Associate Editor‐in‐Chief for Pediatric Anesthesia.
Figures
Similar articles
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase.Br J Clin Pharmacol. 2009 Dec;68(6):920-7. doi: 10.1111/j.1365-2125.2009.03545.x. Br J Clin Pharmacol. 2009. PMID: 20002087 Free PMC article. Clinical Trial.
-
Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: insights from methadone interactions with ritonavir/indinavir.Anesthesiology. 2009 Mar;110(3):660-72. doi: 10.1097/ALN.0b013e3181986a9a. Anesthesiology. 2009. PMID: 19225389 Free PMC article.
-
Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents.Clin Pharmacokinet. 1998 Oct;35(4):275-91. doi: 10.2165/00003088-199835040-00002. Clin Pharmacokinet. 1998. PMID: 9812178 Review.
-
A randomised study of the effect of danoprevir/ritonavir or ritonavir on substrates of cytochrome P450 (CYP) 3A and 2C9 in chronic hepatitis C patients using a drug cocktail.Eur J Clin Pharmacol. 2013 Nov;69(11):1939-49. doi: 10.1007/s00228-013-1556-y. Epub 2013 Jul 20. Eur J Clin Pharmacol. 2013. PMID: 23872824 Clinical Trial.
Cited by
-
Molecular Factors and Pathways of Hepatotoxicity Associated with HIV/SARS-CoV-2 Protease Inhibitors.Int J Mol Sci. 2023 Apr 27;24(9):7938. doi: 10.3390/ijms24097938. Int J Mol Sci. 2023. PMID: 37175645 Free PMC article. Review.
-
Evaluating Drug Interactions between Ritonavir and Opioid Analgesics: Implications from Physiologically Based Pharmacokinetic Simulation.Pharmaceuticals (Basel). 2024 May 15;17(5):640. doi: 10.3390/ph17050640. Pharmaceuticals (Basel). 2024. PMID: 38794210 Free PMC article.
References
-
- Evron S, Glezerman M, Harow E, Sadan O, Ezri T. Human immunodeficiency virus: anesthetic and obstetric considerations. Anesth Analg. 2004;98:503‐511. - PubMed
-
- Schulenburg E, Le Roux PJ. Antiretroviral therapy and anaesthesia. S Afr J Anaesth Analg. 2008;14:31‐38.
-
- Leelanukrom R, Pancharoen C. Anesthesia in HIV‐infected children. Pediatr Anesth. 2007;17:509‐519. - PubMed
-
- Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS‐CoV‐2 M(pro) inhibitor clinical candidate for the treatment of COVID‐19. Science. 2021;374:1586‐1593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

