Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer
- PMID: 35843080
- PMCID: PMC9434408
- DOI: 10.1016/j.esmoop.2022.100527
Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer
Abstract
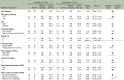
Background: Mature progression-free survival (PFS) data from the phase III J-ALEX study showed superiority for alectinib versus crizotinib [hazard ratio (HR) 0.37, 95% confidence interval (CI) 0.26-0.52; median PFS 34.1 versus 10.2 months, respectively] in advanced ALK (anaplastic lymphoma kinase)-positive non-small-cell lung cancer (NSCLC). Overall survival (OS) data were immature (HR 0.80, 99.8799% CI 0.35-1.82) at the time of data cut-off (30 June 2018). We report final OS data after ≥5 years of follow-up.
Patients and methods: ALK inhibitor naive Japanese patients who were chemotherapy naive or had received one prior chemotherapy regimen were enrolled. Patients were randomized to receive alectinib 300 mg (n = 103) or crizotinib 250 mg (n = 104) twice daily until progressive disease, unacceptable toxicity, death, or withdrawal. The primary endpoint was independent review facility-assessed PFS, with OS (not fully powered) as a secondary endpoint.
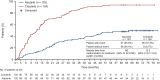
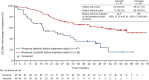
Results: Median duration of OS follow-up was 68.6 months with alectinib and 68.0 months with crizotinib. Treatment with alectinib did not prolong OS relative to crizotinib (HR 1.03, 95.0405% CI 0.67-1.58; P = 0.9105). Five-year OS rates were 60.9% (95% CI 51.4-70.3) with alectinib and 64.1% (95% CI 54.9-73.4) with crizotinib. In total, 91.3% (n = 95/104) of crizotinib-treated patients and 46.6% (n = 48/103) of alectinib-treated patients received at least one subsequent anticancer therapy. After study drug discontinuation, 78.8% of patients in the crizotinib arm switched to alectinib, while 10.7% of patients in the alectinib arm switched to crizotinib as a first subsequent anticancer therapy. Patients randomized to crizotinib tended to switch treatment earlier than those randomized to alectinib.
Conclusion: Final OS analysis from J-ALEX did not show superiority of alectinib to crizotinib; this result was most likely confounded by treatment crossover. Alectinib remains a standard of care for the treatment of patients with advanced ALK-positive NSCLC.
Keywords: ALK-positive NSCLC; J-ALEX; alectinib; crizotinib; overall survival.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Data sharing Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). For further details on Chugai’s Data Sharing Policy and how to request access to related clinical study documents, see here (www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html).
Figures

References
-
- Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. - PubMed
-
- Hanna N.H., Robinson A.G., Temin S., et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39:1040–1091. - PubMed
-
- Hida T., Nokihara H., Kondo M., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. - PubMed
-
- Gadgeel S.M., Gandhi L., Riely G.J., et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. - PubMed
-
- Seto T., Kiura K., Nishio M., et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

