Phosphate position is key in mediating transmembrane ion channel TMEM16A-phosphatidylinositol 4,5-bisphosphate interaction
- PMID: 35843309
- PMCID: PMC9396059
- DOI: 10.1016/j.jbc.2022.102264
Phosphate position is key in mediating transmembrane ion channel TMEM16A-phosphatidylinositol 4,5-bisphosphate interaction
Abstract
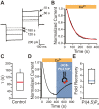
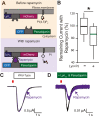
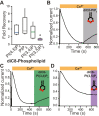
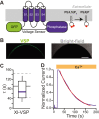
TransMEMbrane 16A (TMEM16A) is a Ca2+-activated Cl- channel that plays critical roles in regulating diverse physiologic processes, including vascular tone, sensory signal transduction, and mucosal secretion. In addition to Ca2+, TMEM16A activation requires the membrane lipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). However, the structural determinants mediating this interaction are not clear. Here, we interrogated the parts of the PI(4,5)P2 head group that mediate its interaction with TMEM16A by using patch- and two-electrode voltage-clamp recordings on oocytes from the African clawed frog Xenopus laevis, which endogenously express TMEM16A channels. During continuous application of Ca2+ to excised inside-out patches, we found that TMEM16A-conducted currents decayed shortly after patch excision. Following this rundown, we show that the application of a synthetic PI(4,5)P2 analog produced current recovery. Furthermore, inducible dephosphorylation of PI(4,5)P2 reduces TMEM16A-conducted currents. Application of PIP2 analogs with different phosphate orientations yielded distinct amounts of current recovery, and only lipids that include a phosphate at the 4' position effectively recovered TMEM16A currents. Taken together, these findings improve our understanding of how PI(4,5)P2 binds to and potentiates TMEM16A channels.
Keywords: anion channel; calcium; chloride channel; oocyte; patch clamp; phospholipid; signal transduction; transmembrane member 16A; xenopus.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Pedemonte N., Galietta L.J. Structure and function of TMEM16 proteins (anoctamins) Physiol. Rev. 2014;94:419–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

