Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design
- PMID: 35843374
- PMCID: PMC9974107
- DOI: 10.1016/j.jhep.2022.07.004
Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design
Abstract
Background & aims: The predicted risk and timeline to progression to liver-related outcomes in the population with NAFLD are not well-characterized. We aimed to examine the risk and time to progression to cirrhosis, hepatic decompensation and death in a contemporary population over a long follow-up period, to obtain information to guide endpoint selection and sample size calculations for clinical trials on NAFLD-related cirrhosis.
Methods: This is a retrospective study of prospectively collected data in a medical record linkage system, including all adults diagnosed with NAFLD between 1996-2016 by clinical, biochemical and radiological criteria in Olmsted County, Minnesota and followed until 2019. Liver-related outcomes and death were ascertained and validated by individual medical record review. Time and risk of progression from NAFLD to cirrhosis to decompensation and death were assessed using multistate modeling.
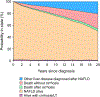
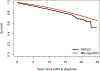
Results: A total of 5,123 individuals with NAFLD (median age 52 years, 53% women) were followed for a median of 6.4 (range 1-23) years. The risk of progression was as follows: from NAFLD to cirrhosis: 3% in 15 years; compensated cirrhosis to first decompensation: 33% in 4 years (8%/year); first decompensation to ≥2 decompensations: 48% in 2 years. Albumin, bilirubin, non-bleeding esophageal varices and diabetes were independent predictors of decompensation. Among the 575 deaths, 6% were liver related. Therapeutic trials in compensated cirrhosis would require enrolment of a minimum of 2,886 individuals followed for >2 years to detect at least a 15% relative decrease in liver-related endpoints.
Conclusion: In this population-based cohort with 23 years of longitudinal follow-up, NAFLD was slowly progressive, with liver-related outcomes affecting only a small proportion of people. Large sample sizes and long follow-up are required to detect reductions in liver-related endpoints in clinical trials.
Lay summary: For patients with compensated non-alcoholic steatohepatitis-related cirrhosis, the time spent in this state and the risk of progression to decompensation are not well-known in the population. We examined the clinical course of a large population-based cohort over 23 years of follow-up. We identified that adults with compensated cirrhosis spend a mean time of 4 years in this state and have a 10% per year risk of progression to decompensation or death. The risk of further progression is 3-fold higher in adults with cirrhosis and one decompensating event. These results are reflective of placebo arm risks in drug clinical trials and are essential in the estimation of adequate sample sizes.
Keywords: MAFLD; cirrhosis; endpoints; outcomes; progression.
Copyright © 2022 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest All the authors disclose no conflicts. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



Comment in
-
Portal hypertension is a key determinant of the risk for liver-related events in non-alcoholic fatty liver disease.J Hepatol. 2023 Mar;78(3):e102-e104. doi: 10.1016/j.jhep.2022.08.031. Epub 2022 Sep 5. J Hepatol. 2023. PMID: 36070837 No abstract available.
-
NASH cirrhosis trials and major adverse liver outcomes: Big data needed.J Hepatol. 2023 Jan;78(1):5-7. doi: 10.1016/j.jhep.2022.10.022. Epub 2022 Oct 31. J Hepatol. 2023. PMID: 36328331 No abstract available.
References
-
- Rinella ME, Tacke F, Sanyal AJ, Anstee QM. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology 2019;70:1424–1436. - PubMed
-
- Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, Paradis V, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol 2021. - PubMed
-
- Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, Shiftman ML, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology 2019;70:1913–1927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

