SARS-CoV-2 and the central nervous system: Emerging insights into hemorrhage-associated neurological consequences and therapeutic considerations
- PMID: 35843590
- PMCID: PMC9288264
- DOI: 10.1016/j.arr.2022.101687
SARS-CoV-2 and the central nervous system: Emerging insights into hemorrhage-associated neurological consequences and therapeutic considerations
Abstract
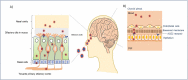
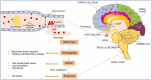
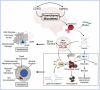
Coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) continues to impact our lives by causing widespread illness and death and poses a threat due to the possibility of emerging strains. SARS-CoV-2 targets angiotensin-converting enzyme 2 (ACE2) before entering vital organs of the body, including the brain. Studies have shown systemic inflammation, cellular senescence, and viral toxicity-mediated multi-organ failure occur during infectious periods. However, prognostic investigations suggest that both acute and long-term neurological complications, including predisposition to irreversible neurodegenerative diseases, can be a serious concern for COVID-19 survivors, especially the elderly population. As emerging studies reveal sites of SARS-CoV-2 infection in different parts of the brain, potential causes of chronic lesions including cerebral and deep-brain microbleeds and the likelihood of developing stroke-like pathologies increases, with critical long-term consequences, particularly for individuals with neuropathological and/or age-associated comorbid conditions. Our recent studies linking the blood degradation products to genome instability, leading to cellular senescence and ferroptosis, raise the possibility of similar neurovascular events as a result of SARS-CoV-2 infection. In this review, we discuss the neuropathological consequences of SARS-CoV-2 infection in COVID survivors, focusing on possible hemorrhagic damage in brain cells, its association to aging, and the future directions in developing mechanism-guided therapeutic strategies.
Keywords: Brain fog; COVID-19; Coronavirus; Ferroptosis; Genome instability; Hemorrhage; SARS-CoV-2; Senescence.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ahn J.H., Kim J., Hong S.P., Choi S.Y., Yang M.J., Ju Y.S., Kim Y.T., Kim H.M., Rahman M.D.T., Chung M.K., Hong S.D., Bae H., Lee C.S., Koh G.Y. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J. Clin. Investig. 2021;131(13) doi: 10.1172/jci148517. - DOI - PMC - PubMed
-
- Ahn M., Anderson D.E., Zhang Q., Tan C.W., Lim B.L., Luko K., Wen M., Chia W.N., Mani S., Wang L.C., Ng J.H.J., Sobota R.M., Dutertre C.A., Ginhoux F., Shi Z.L., Irving A.T., Wang L.F. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019;4(5):789–799. doi: 10.1038/s41564-019-0371-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

