What Causes Hypertrophic Cardiomyopathy?
- PMID: 35843734
- PMCID: PMC9818026
- DOI: 10.1016/j.amjcard.2022.06.017
What Causes Hypertrophic Cardiomyopathy?
Abstract
Hypertrophic cardiomyopathy (HCM) is a global and relatively common cause of patient morbidity and mortality and is among the first reported monogenic cardiac diseases. For 30 years, the basic etiology of HCM has been attributed largely to variants in individual genes encoding cardiac sarcomere proteins, with the implication that HCM is fundamentally a genetic disease. However, data from clinical and network medicine analyses, as well as contemporary genetic studies show that single gene variants do not fully explain the broad and diverse HCM clinical spectrum. These transformative advances place a new focus on possible novel interactions between acquired disease determinants and genetic context to produce complex HCM phenotypes, also offering a measure of caution against overemphasizing monogenics as the principal cause of this disease. These new perspectives in which HCM is not a uniformly genetic disease but likely explained by multifactorial etiology will also unavoidably impact how HCM is viewed by patients and families in the clinical practicing community going forward, including relevance to genetic counseling and access to healthcare insurance and psychosocial wellness.
Published by Elsevier Inc.
Figures

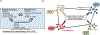
References
-
- Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018;379:655–668. - PubMed
-
- Maron BJ, Rowin EJ, Casey SA, Maron. How hypertrophic cardiomyopathy became a contemporary and treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol 2016;1:98–105. - PubMed
-
- De Marvao A, McGurk KA, Zheng SL, Thanaj M, Bai W, Duan J, Biffi C, Mazzarotto F, Statton B, Dawes TJW, Savioli N, Halliday BP, Xu X, Buchan RJ, Baksi AJ, Quinlan M, Tokarczuk P, Tayal U, Francis C, Whiffin N, Theotokis PI, Zhang X, Jang M, Berry A, Pantazis A, Barton PJR, Rueckert D, Prasad SK, Walsh R, Ho CY, Cook SA, Ware JS, O’Regan DP. Phenotypic expression and outcomes in individuals with rare genetic variants of hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:1097–1110. - PMC - PubMed
-
- Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Eliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HVHV, Semsarian C, Sorajja P, Members ACC/AHA Joint Committee, O'Gara PT, Beckman JA, Levine GN, Al-Khatib SM, Armbruster A, Birtcher KK, Ciggaroa J, Dixon DL, de Las Fuentes L, Deswal A, Fleisher LA, Gentile F, Goldberger ZD, Gorenek B, Haynes N, Hernandez AF, Hlatky MA, Joglar JA, Jones WS, Marine JE, Mark D, Palaniappan L, Piano MR, Tamis-Holland J, Wijeysundera DN, Woo YJ. 2020 AHA/ACC Guidelines for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2020;76:3022–3055. - PubMed
-
- Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

