Optimization of the production process for the anticancer lead compound illudin M: process development in stirred tank bioreactors
- PMID: 35843931
- PMCID: PMC9290264
- DOI: 10.1186/s12934-022-01870-w
Optimization of the production process for the anticancer lead compound illudin M: process development in stirred tank bioreactors
Abstract
Background: The fungal natural products illudin S and M have been investigated as precursors for the development of semisynthetic anticancer agents such as Irofulven (illudin S derivative) which is currently in phase II clinical trials. Recently, illudin M derivatives have shown improved in vitro selectivity towards cancer cells encouraging further investigation. This requires a stable supply of the precursor which is produced by Basidiomycota of the genus Omphalotus. We have recently reported a robust shake flask process for the production of gram quantities of illudin M from Omphalotus nidiformis aiming to transfer that process into stirred tank bioreactors, which can be used in a commercial production set-up. However, process transfer across different systems is not straightforward and particularly challenging when the producer is morphologically complex. There are only a few reports that address the development of bioprocesses for the production of compounds from Basidiomycota as these organisms have not been extensively studied because of their complex life cycles and often are difficult to cultivate under laboratory conditions.
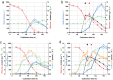
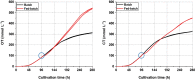
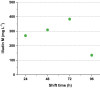
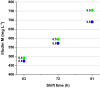
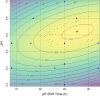
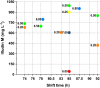
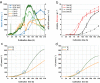
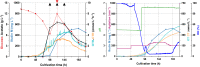
Results: The recently developed shake flask process delivering stable titers of ~ 940 mg L-1 of illudin M was investigated using off-gas analysis to identify critical parameters which facilitated the transfer from shaken into stirred tank bioreactors. Comparable titers to the shake flask process were achieved in 2 L stirred tank bioreactors (1.5 L working volume) by controlling growth of biomass with a carefully timed pH-shift combined with an improved precursor-feeding strategy. A scale-up experiment in a 15 L bioreactor (10 L working volume), resembling the process at 1.5 L resulted in 523 mg L-1 and is the starting point for optimization of the identified parameters at that scale.
Conclusion: By identifying and controlling key process parameters, the production process for illudin M was transferred from shake flasks into 2 L stirred tank bioreactors reaching a comparable titer (> 900 mg L-1), which is significantly higher than any previously reported. The insights obtained from 10 L scale pave the way towards further scale-up studies that will enable a sustainable supply of illudin M to support preclinical and clinical development programs.
Keywords: Acetate feed; Anticancer molecules; Basidiomycota; Bioprocess optimization; DASGIP; Fungal biotechnology; Natural products; RAMOS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Optimization of the production process for the anticancer lead compound illudin M: downstream processing.Microb Cell Fact. 2022 Aug 17;21(1):165. doi: 10.1186/s12934-022-01886-2. Microb Cell Fact. 2022. PMID: 35978411 Free PMC article.
-
Optimization of the production process for the anticancer lead compound illudin M: improving titers in shake-flasks.Microb Cell Fact. 2022 May 28;21(1):98. doi: 10.1186/s12934-022-01827-z. Microb Cell Fact. 2022. PMID: 35643529 Free PMC article.
-
Validation of the transferability of membrane-based fed-batch shake flask cultivations to stirred-tank reactor using three different protease producing Bacillus strains.J Biosci Bioeng. 2019 Nov;128(5):599-605. doi: 10.1016/j.jbiosc.2019.05.003. Epub 2019 Jun 20. J Biosci Bioeng. 2019. PMID: 31151898
-
Parallel reactor systems for bioprocess development.Adv Biochem Eng Biotechnol. 2005;92:125-43. doi: 10.1007/b98916. Adv Biochem Eng Biotechnol. 2005. PMID: 15791935 Review.
-
Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview.Biotechnol Adv. 2009 Mar-Apr;27(2):153-76. doi: 10.1016/j.biotechadv.2008.10.006. Epub 2008 Nov 12. Biotechnol Adv. 2009. PMID: 19041387 Review.
Cited by
-
Optimization of the production process for the anticancer lead compound illudin M: downstream processing.Microb Cell Fact. 2022 Aug 17;21(1):165. doi: 10.1186/s12934-022-01886-2. Microb Cell Fact. 2022. PMID: 35978411 Free PMC article.
-
Spore-Derived Isolates from a Single Basidiocarp of Bioluminescent Omphalotus olivascens Reveal Multifaceted Phenotypic and Physiological Variations.Microorganisms. 2025 Jan 1;13(1):59. doi: 10.3390/microorganisms13010059. Microorganisms. 2025. PMID: 39858827 Free PMC article.
-
Fungi: Pioneers of chemical creativity - Techniques and strategies to uncover fungal chemistry.IMA Fungus. 2025 Mar 7;16:e142462. doi: 10.3897/imafungus.16.142462. eCollection 2025. IMA Fungus. 2025. PMID: 40093757 Free PMC article. Review.
-
Optimization of the production process for the anticancer lead compound illudin M: improving titers in shake-flasks.Microb Cell Fact. 2022 May 28;21(1):98. doi: 10.1186/s12934-022-01827-z. Microb Cell Fact. 2022. PMID: 35643529 Free PMC article.
References
-
- Allarity Therapeutics. Pipeline: Clinical Trials. 2020. https://allarity.com/pipeline/clinical-trials/. Accessed Aug 9 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

