Pharmacokinetic Characteristics, Safety, and Tolerability of Telitacicept, an Injectable Recombinant Human B-Lymphocyte Stimulating Factor Receptor-Antibody Fusion Protein, in Healthy Chinese Subjects
- PMID: 35844038
- PMCID: PMC9796261
- DOI: 10.1002/cpdd.1136
Pharmacokinetic Characteristics, Safety, and Tolerability of Telitacicept, an Injectable Recombinant Human B-Lymphocyte Stimulating Factor Receptor-Antibody Fusion Protein, in Healthy Chinese Subjects
Abstract
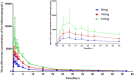
Telitacicept, an injectable recombinant human B-lymphocyte stimulating factor receptor-antibody fusion protein, is a new dual B lymphocyte stimulator (BLyS)/APRIL (a proliferation-inducing ligand) inhibitor that effectively blocks proliferation of B lymphocytes. This study evaluates the pharmacokinetic characteristics, tolerability, and safety of a single subcutaneous injection of various doses (80, 160, and 240 mg) of telitacicept in healthy Chinese subjects. This trial is a single-center, randomized, open-label phase I clinical study that includes three dose groups (80, 160, and 240 mg) with 12 subjects in each dose group. The subjects were randomly assigned to different dose groups in a 1:1:1 ratio for a single subcutaneous administration trial. After a single dose, the maximum serum concentration (Cmax ) of total and free telitacicept was reached within 0.5-1 days. The elimination half-lives of total and free telitacicept at doses of 80-240 mg were 10.9-11.9 days and 11-12.5 days, respectively. The formation and elimination of the BLyS-telitacicept complex were much slower; the median time to Cmax was 15-57 days and was significantly prolonged with increasing dose. Only two of the 36 healthy subjects had positive antidrug antibodies with antibody titers of 1:15. The severity of adverse events was mild or moderate, and no higher treatment-emergent adverse events were reported. In conclusion, total telitacicept within a dose range of 80-240 mg and free telitacicept within a dose range of 160-240 mg had linear pharmacokinetic characteristics.
Keywords: BLyS/APRIL; pharmacokinetics; safety; telitacicept; tolerability.
© 2022 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures



References
-
- Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63‐80. - PubMed
-
- Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045‐1051. - PubMed
-
- Smolen, JS , Landewé R, Bijlsma J, et al. EULAR recommendation for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79 (6):685‐699. - PubMed
-
- Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125(4):814‐820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

