Efficacy and tolerability of brivaracetam monotherapy in childhood and juvenile absence epilepsy: An innovative adaptive trial design
- PMID: 35844134
- PMCID: PMC9712476
- DOI: 10.1002/epi4.12628
Efficacy and tolerability of brivaracetam monotherapy in childhood and juvenile absence epilepsy: An innovative adaptive trial design
Abstract
Objective: Despite introduction of several antiseizure medications over the past two decades, treatment options for childhood absence epilepsy (CAE) and juvenile absence epilepsy (JAE) remain limited. We report the innovative adaptive design of an ongoing phase 2/3 trial to evaluate efficacy, safety, and tolerability of brivaracetam (BRV) monotherapy in patients 2-25 years of age with CAE or JAE.
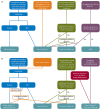
Methods: N01269 (ClinicalTrials.gov: NCT04666610; start: July 2021; expected completion: 2024) is a randomized, dose-finding and confirmatory, double-blind, placebo-controlled, parallel-group, multicenter trial. The trial consists of a dose-selection and assessment for futility stage, followed by an optimal-dose stage after interim analysis. Both stages include an up to 2-week screening period, a 2-week placebo-controlled period, and an 11-week active treatment period (10 weeks of initial treatment followed by a 24-hour electroencephalogram [EEG] and an additional week of active treatment for 24-hour EEG assessment). Patients who are absence seizure-free will enter an up to 4-week randomized withdrawal period. Efficacy assessments will be based on 24-hour EEG and seizure diaries.
Significance: This two-stage adaptive trial design allows investigation of two potentially efficacious BRV doses, where one dose is dropped in favor of the other dose with a better benefit-risk profile. This allows for a combined phase 2 dose-finding and phase 3 confirmatory efficacy trial, which reduces the number of patients needed to be recruited and reduces trial duration. A randomized withdrawal period is included to evaluate sustainability of treatment effect over time and to allow for placebo control while minimizing placebo exposure. Use of EEG capture in addition to seizure diaries offers a robust mechanism of detecting seizure activity and measuring treatment effect. Positive efficacy and safety/tolerability data may support the use of BRV as monotherapy for CAE or JAE, providing another treatment option and representing long-delayed progress in the treatment of absence seizures in these populations.
Keywords: antiseizure medication; double-blind placebo-controlled; electroencephalogram; randomized withdrawal; seizures.
© 2022 UCB Biosciences GmbH. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Thomas Bast has received support from Bial, Desitin Arzneimittel GmbH, Eisai, GW Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Shire, Takeda, UCB Pharma, and Zogenix. Anne‐Liv Schulz, Florin Floricel, Diego Morita, Jody Cleveland, and Jan‐Peer Elshoff are salaried employees of UCB Pharma and have received stock or stock options from their employment.
Figures
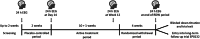

References
-
- Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. - PubMed
-
- Asadi‐Pooya AA, Emami M, Sperling MR. A clinical study of syndromes of idiopathic (genetic) generalized epilepsy. J Neurol Sci. 2013;324:113–7. - PubMed
-
- Trudeau V, Myers S, LaMoreaux L, Anhut H, Garofalo E, Ebersole J. Gabapentin in naive childhood absence epilepsy: results from two double‐blind, placebo‐controlled, multicenter studies. J Child Neurol. 1996;11:470–5. - PubMed
-
- Frank LM, Enlow T, Holmes GL, Manasco P, Concannon S, Chen C, et al. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia. 1999;40:973–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

