Antipsychotics for schizophrenia spectrum disorders with catatonic symptoms
- PMID: 35844143
- PMCID: PMC9289703
- DOI: 10.1002/14651858.CD013100.pub2
Antipsychotics for schizophrenia spectrum disorders with catatonic symptoms
Abstract
Background: Whilst antipsychotics are the mainstay of treatment for schizophrenia spectrum disorders, there have been numerous attempts to identify biomarkers that can predict treatment response. One potential marker may be psychomotor abnormalities, including catatonic symptoms. Early studies suggested that catatonic symptoms predict poor treatment response, whilst anecdotal reports of rare adverse events have been invoked against antipsychotics. The efficacy and safety of antipsychotics in the treatment of this subtype of schizophrenia have rarely been studied in randomised controlled trials (RCTs).
Objectives: To compare the effects of any single antipsychotic medication with another antipsychotic or with other pharmacological agents, electroconvulsive therapy (ECT), other non-pharmacological neuromodulation therapies (e.g. transcranial magnetic stimulation), or placebo for treating positive, negative, and catatonic symptoms in people who have schizophrenia spectrum disorders with catatonic symptoms.
Search methods: We searched the Cochrane Schizophrenia Group's Study-Based Register of Trials, which is based on CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, PubMed, ClinicalTrials.gov, the ISRCTN registry, and WHO ICTRP, on 19 September 2021. There were no language, date, document type, or publication status limitations for inclusion of records in the register. We also manually searched reference lists from the included studies, and contacted study authors when relevant.
Selection criteria: All RCTs comparing any single antipsychotic medication with another antipsychotic or with other pharmacological agents, ECT, other non-pharmacological neuromodulation therapies, or placebo for people who have schizophrenia spectrum disorders with catatonic symptoms.
Data collection and analysis: two review authors independently inspected citations, selected studies, extracted data, and appraised study quality. For binary outcomes, we planned to calculate risk ratios and their 95% confidence intervals (CI) on an intention-to-treat basis. For continuous outcomes, we planned to calculate mean differences between groups and their 95% CI. We assessed risk of bias for the included studies, and created a summary of findings table; however, we did not assess the certainty of the evidence using the GRADE approach because there was no quantitative evidence in the included study.
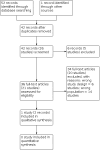
Main results: Out of 53 identified reports, one RCT including 14 hospitalised adults with schizophrenia and catatonic symptoms met the inclusion criteria of the review. The study, which was conducted in India and lasted only three weeks, compared risperidone with ECT in people who did not respond to an initial lorazepam trial. There were no usable data reported on the primary efficacy outcomes of clinically important changes in positive, negative, or catatonic symptoms. Whilst both study groups improved in catatonia scores on the Bush-Francis Catatonia Rating Scale (BFCRS), the ECT group showed significantly greater improvement at week 3 endpoint (mean +/- estimated standard deviation; 0.68 +/- 4.58; N = 8) than the risperidone group (6.04 +/- 4.58; N = 6; P = 0.035 of a two-way analysis of variance (ANOVA) for repeated measures originally conducted in the trial). Similarly, both groups improved on the Positive and Negative Syndrome Scale (PANSS) scores by week 3, but ECT showed significantly greater improvement in positive symptoms scores compared with risperidone (P = 0.04). However, data on BFCRS scores in the ECT group appeared to be skewed, and mean PANSS scores were not reported, thereby precluding further analyses of both BFCRS and PANSS data according to the protocol. Although no cases of neuroleptic malignant syndrome were reported, extrapyramidal symptoms as a primary safety outcome were reported in three cases in the risperidone group. Conversely, headache (N = 6), memory loss (N = 4), and a prolonged seizure were reported in people receiving ECT. These adverse effects, which were assessed as specific for antipsychotics and ECT, respectively, were the only adverse effects reported in the study. However, the exact number of participants with adverse events was not clearly reported in both groups, precluding further analysis. Our results were based only on a single study with a very small sample size, short duration of treatment, unclear or high risk of bias due to unclear randomisation methods, possible imbalance in baseline characteristics, skewed data, and selective reporting. Data on outcomes of general functioning, global state, quality of life, and service use, as well as data on specific phenomenology and duration of catatonic symptoms, were not reported.
Authors' conclusions: We found only one small, short-term trial suggesting that risperidone may improve catatonic and positive symptoms scale scores amongst people with schizophrenia spectrum disorders and catatonic symptoms, but that ECT may result in greater improvement in the first three weeks of treatment. Due to small sample size, methodological shortcomings and brief duration of the study, as well as risk of bias, the evidence from this review is of very low quality. We are uncertain if these are true effects, limiting any conclusions that can be drawn from the evidence. No cases of neuroleptic malignant syndrome were reported, but we cannot rule out the risk of this or other rare adverse events in larger population samples. High-quality trials continue to be necessary to differentiate treatments for people with symptoms of catatonia in schizophrenia spectrum disorders. The lack of consensus on the psychopathology of catatonia remains a barrier to defining treatments for people with schizophrenia. Better understanding of the efficacy and safety of antipsychotics may clarify treatment for this unique subtype of schizophrenia.
Antecedentes: Aunque los antipsicóticos son la base del tratamiento de los trastornos del espectro de la esquizofrenia, ha habido numerosos intentos de identificar biomarcadores que puedan predecir la respuesta al tratamiento. Un posible marcador podrían ser las anomalías psicomotoras, incluidos los síntomas catatónicos. Los estudios más antiguos indican que los síntomas catatónicos predicen una respuesta deficiente al tratamiento, mientras que se han alegado informes anecdóticos de eventos adversos poco frecuentes contra los antipsicóticos. La eficacia y la seguridad de los antipsicóticos en el tratamiento de este subtipo de esquizofrenia rara vez se han estudiado en ensayos controlados aleatorizados (ECA).
Objetivos: Comparar los efectos de cualquier fármaco antipsicótico único con otro antipsicótico o con otros agentes farmacológicos, terapia electroconvulsiva (TEC), otras terapias de neuromodulación no farmacológicas (p. ej., estimulación magnética transcraneal) o placebo para el tratamiento de los síntomas positivos, negativos y catatónicos en personas que presentan trastornos del espectro de la esquizofrenia con síntomas catatónicos. MÉTODOS DE BÚSQUEDA: El 19 de septiembre de 2021 se realizaron búsquedas en el registro de ensayos basados en estudios del Grupo Cochrane de Esquizofrenia (Cochrane Schizophrenia Group), que se basa en CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, PubMed, ClinicalTrials.gov, el registro ISRCTN y la ICTRP de la OMS. No hubo limitaciones de idioma, fecha, tipo de documento o estado de publicación para la inclusión de los registros en el registro. También se realizaron búsquedas manuales en las listas de referencias de los estudios incluidos y se estableció contacto con los autores de los estudios cuando fue pertinente. CRITERIOS DE SELECCIÓN: Todos los ECA que compararan cualquier fármaco antipsicótico único con otro antipsicótico o con otros agentes farmacológicos, TEC, otras terapias de neuromodulación no farmacológicas o placebo en personas que presentan trastornos del espectro de la esquizofrenia con síntomas catatónicos. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión inspeccionaron de forma independiente las citas, seleccionaron los estudios, extrajeron los datos y evaluaron la calidad de los estudios. Para los desenlaces binarios se planeó calcular las razones de riesgos y sus intervalos de confianza (IC) del 95% sobre la base de la intención de tratar. Para los desenlaces continuos se planeó calcular las diferencias de medias entre los grupos y sus IC del 95%. Se evaluó el riesgo de sesgo de los estudios incluidos y se creó una tabla de resumen de los hallazgos. Sin embargo, no se evaluó la certeza de la evidencia mediante el método GRADE porque no hubo evidencia cuantitativa en el estudio incluido.
Resultados principales: De los 53 informes identificados, un ECA que incluyó a 14 adultos hospitalizados con esquizofrenia y síntomas catatónicos cumplió con los criterios de inclusión de la revisión. El estudio, realizado en la India y que sólo duró tres semanas, comparó la risperidona con la TEC en personas que no respondieron a una prueba inicial con lorazepam. No se informaron datos utilizables sobre los desenlaces principales de eficacia de cambios clínicamente importantes en los síntomas positivos, negativos o catatónicos. Aunque ambos grupos del estudio mejoraron en las puntuaciones de catatonia en la Bush‐Francis Catatonia Rating Scale (BFCRS), el grupo de TEC mostró una mejoría significativamente mayor en el desenlace a las tres semanas (media +/‐ desviación estándar estimada; 0,68 +/‐ 4,58; n = 8) que el grupo de risperidona (6,04 +/‐ 4,58; n = 6; p = 0,035 de un análisis de varianza (ANOVA) de dos vías para medidas repetidas realizado originalmente en el ensayo). Asimismo, ambos grupos mejoraron en las puntuaciones de la Positive and Negative Syndrome Scale (PANSS) a las tres semanas, pero la TEC mostró una mejoría significativamente mayor en las puntuaciones de los síntomas positivos en comparación con la risperidona (p = 0,04). Sin embargo, los datos sobre las puntuaciones de la BFCRS en el grupo de TEC parecieron estar sesgados, y no se informaron las puntuaciones medias de la PANSS, lo que impidió realizar más análisis de los datos de la BFCRS y la PANSS según el protocolo. Aunque no se informaron casos de síndrome neuroléptico maligno, en tres casos del grupo de risperidona se notificaron síntomas extrapiramidales como un desenlace principal de seguridad. Por el contrario, en las personas que recibieron TEC se informó cefalea (n = 6), pérdida de memoria (n = 4) y una convulsión prolongada. Estos efectos adversos, que se evaluaron como específicos de los antipsicóticos y de la TEC, respectivamente, fueron los únicos efectos adversos notificados en el estudio. Sin embargo, el número exacto de participantes con eventos adversos no se informó claramente en ambos grupos, lo que impidió realizar un análisis más profundo. Los resultados de esta revisión se basaron en un solo estudio con un tamaño muestral muy pequeño, una duración corta del tratamiento, un riesgo de sesgo incierto o alto debido a métodos de asignación al azar poco claros, un posible desequilibrio en las características iniciales, datos sesgados y un informe selectivo. No se informaron datos sobre los desenlaces de funcionalidad general, estado global, calidad de vida ni uso de los servicios, así como tampoco datos sobre la fenomenología específica ni la duración de los síntomas catatónicos.
Conclusiones de los autores: Solo se encontró un ensayo pequeño, a corto plazo, que indica que la risperidona podría mejorar las puntuaciones de la escala de síntomas catatónicos y positivos entre las personas con trastornos del espectro de la esquizofrenia y síntomas catatónicos, pero que la TEC podría producir una mayor mejoría en las primeras tres semanas de tratamiento. Debido al pequeño tamaño muestral, las deficiencias metodológicas y la breve duración del estudio, así como el riesgo de sesgo, la evidencia de esta revisión es de calidad muy baja. No hay confianza en que estos efectos sean verdaderos, lo que limita cualquier conclusión que se pueda sacar a partir de la evidencia. No se notificaron casos de síndrome neuroléptico maligno, pero no se puede descartar el riesgo de este u otros eventos adversos poco frecuentes en muestras poblacionales más grandes. Aún se necesitan ensayos de calidad alta para diferenciar los tratamientos en las personas con síntomas de catatonia en los trastornos del espectro de la esquizofrenia. La falta de consenso sobre la psicopatología de la catatonia todavía es un obstáculo para definir los tratamientos para las personas con esquizofrenia. Un mejor conocimiento de la eficacia y la seguridad de los antipsicóticos podría aclarar el tratamiento de este subtipo único de esquizofrenia.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MH: none known.
RG: received speaker fee from Roche and payments for travel and registration to attend a professional conference from Janssen Pharmaceuticals.
MJ: Co‐ordinating Editor of Cochrane Schizophrenia. MJ has excluded himself entirely from the editorial process of this review.
SC: Consultant to Neurocrine Biosciences and TEVA Pharmaceutical Industries; received research grant support for unrelated projects from Neurocrine Biosciences, Osmotica Pharmaceuticals, and Eagle Pharmaceuticals.
Figures
Update of
- doi: 10.1002/14651858.CD013100
Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Selective noradrenaline reuptake inhibitors for schizophrenia.Cochrane Database Syst Rev. 2018 Jan 25;1(1):CD010219. doi: 10.1002/14651858.CD010219.pub2. Cochrane Database Syst Rev. 2018. PMID: 29368813 Free PMC article.
-
Risperidone (depot) for schizophrenia.Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD004161. doi: 10.1002/14651858.CD004161.pub2. Cochrane Database Syst Rev. 2016. PMID: 27078222 Free PMC article.
-
Atypical antipsychotics for psychosis in adolescents.Cochrane Database Syst Rev. 2013 Oct 15;2013(10):CD009582. doi: 10.1002/14651858.CD009582.pub2. Cochrane Database Syst Rev. 2013. PMID: 24129841 Free PMC article.
-
Cannabis and schizophrenia.Cochrane Database Syst Rev. 2014 Oct 14;2014(10):CD004837. doi: 10.1002/14651858.CD004837.pub3. Cochrane Database Syst Rev. 2014. PMID: 25314586 Free PMC article.
Cited by
-
Catatonia-related adverse outcomes after long-acting injectable antipsychotics: Case series.SAGE Open Med Case Rep. 2024 Jan 31;12:2050313X241229008. doi: 10.1177/2050313X241229008. eCollection 2024. SAGE Open Med Case Rep. 2024. PMID: 38304856 Free PMC article.
-
Plasmapheresis for a Patient with Catatonia and Systemic Lupus Erythematosus: A Case Report and Literature Review.J Clin Med. 2022 Nov 10;11(22):6670. doi: 10.3390/jcm11226670. J Clin Med. 2022. PMID: 36431144 Free PMC article.
-
The efficacy and safety of sodium nitroprusside in the treatment of schizophrenia: a meta-analysis.Front Psychiatry. 2023 Nov 6;14:1271624. doi: 10.3389/fpsyt.2023.1271624. eCollection 2023. Front Psychiatry. 2023. PMID: 38025431 Free PMC article.
-
Spontaneous Bladder Rupture in a Catatonic Schizophrenia Patient.Case Rep Psychiatry. 2023 Nov 21;2023:4277372. doi: 10.1155/2023/4277372. eCollection 2023. Case Rep Psychiatry. 2023. PMID: 38033476 Free PMC article.
-
Use of First-Generation Antipsychotics in an Adolescent Male with Catatonic Schizophrenia.Harv Rev Psychiatry. 2023 Nov-Dec 01;31(6):267-273. doi: 10.1097/HRP.0000000000000381. Epub 2023 Oct 13. Harv Rev Psychiatry. 2023. PMID: 37823777 Free PMC article. No abstract available.
References
References to studies included in this review
Girish 2003 {published data only (unpublished sought but not used)}
-
- Kunigiri G, Gill NS, B GN, Naimmagadda J. Electroconvulsive therapy in lorazepam non-responsive catatonia. In: 12th World Congress of Psychiatry; 2002 Aug 24-29; Yokohama, Japan. 2002 .
References to studies excluded from this review
Alexander 1978 {published data only}
-
- Alexander PE, Van Kammen DP, Bunney WE. Serum calcium and magnesium in schizophrenia: relationship to clinical phenomena and neuroleptic treatment. British Journal of Psychiatry 1978;133:143-9. - PubMed
Athanassenas 1983 {published data only}
-
- Athanassenas G, Papadopoulos E, Kourkoubas A, Tsitourides S, Gabriel J, Hoidas S, et al. Serum calcium and magnesium levels in chronic schizophrenics. Journal of Clinical Psychopharmacology 1983;3(4):212-6. - PubMed
Bagadia 1983 {published data only}
-
- Bagadia VN, Abhyanak RR, Doshi J, Pradhan PV, Shah LP. A double blind controlled study of ECT vs chlorpromazine in schizophrenia. JAPI: Journal of the Association of Physicians of India 1983;31:637-40. - PubMed
Ceskova 1994 {published data only}
-
- Ceskova E, Svestka J. Efficacy and tolerability of risperidone in different dose levels. Neuropyschopharmacology 1994;10(3, 2):193.
-
- Ceskova E, Svestka J. Efficacy and tolerability of risperidone in different dose levels [Ucinnost a snasenlivost risperidonu pri ruznem davkovani (sdeleni z praxe)]. Ceskoslovenska Psychiatrie 1996;92(1):50-6. - PubMed
-
- Ceskova E, Svetska J. Risperidone vs. perphenazine - a double-blind comparison and prolactin plasma levels. Psychiatria Danubina 1994;6(3-4):151-5.
CTRI‐2010‐091‐001409 {published data only}
-
- CTRI-2010-091-001409. A clinical trial to study prevention of relapse in patients with schizophrenia receiving either flexible dose iloperidone or placebo in long-term use. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2010/091/001409 (first received 7 December 2018).
Cuesta 2009 {published and unpublished data}
-
- Cuesta MJ, De Jalon EG, Campos MS, Peralta V. Cognitive effectiveness of olanzapine and risperidone in first-episode psychosis. British Journal of Psychiatry 2009;194(5):439-45. - PubMed
Denef 1971 {published data only}
-
- Denef P, Baro F, Van Lommel R, Dom R. Treatment of chronic psychotic patients with high doses of propericiazine. In: 5th World Congress of Psychiatry; 1971 Nov 28-Dec 4; Mexico City. 1971:262-3.
Drummond 1996 {published data only}
-
- AstraZeneca. A multicentre, double-blind, randomised trial to compare the effects of quetiapine and haloperidol treatment strategies on treatment outcomes (5077IL/0050 ESTO). (This study was accessed for the Cochrane database on 9 April 2001 from a web-based repository of pharmaceutical industry clinical trials (www.clinicalstudyresults.org/), which was established by the Pharmaceutical Research and Manufacturers of America but was phased out 20 December 2011.) (first received 7 December 2018).
-
- Drummond M, Knapp M, Burns T, Miller K, Ruiz R. Methodological issues in the outcomes assessment of a new, atypical antipsychotic. In: 9th European College of Neuropsychopharmacology Congress; 1996 Sep 21-25; Amsterdam. 1996.
-
- Drummond MF, Knapp MR, Burns TP, Shadwell P. Issues in the design of studies for the economic evaluation of new atypical antipsychotics: the ESTO study. Journal of Mental Health Policy and Economics 1998;1(1):15-22. - PubMed
Fischer‐Cornelssen 1976 {published data only}
-
- Dick P, Remy M, Rey Bellet JJ. Comparison of two antipsychotic drugs: chlorpromazine and clozapine [Essai de comparaison de deux antipsychotiques: la chlorpromazine et la clozapine]. Therapeutische Umschau. Revue Therapeutique 1975;32(8):497-500. - PubMed
-
- Ekblom B, Haggstrom JE. Clozapine (leponex) compared with chlorpromazine - a double blind evaluation of pharmacological and clinical properties. Current Therapeutic Research, Clinical and Experimental 1974;16(9):945-57. - PubMed
-
- Fischer Cornelssen K, Ferner U, Steiner H. Multifocal psychopharmaceutic testing ("Multihospital trial") [Multifokale Psychopharmakaprufung ("Multihospital Trial")]. Arzneimittelforschung 1974;24(10):1706-24. - PubMed
-
- Fischer-Cornelssen K, Ferner U. Results of European multi-center double-blind studies with clozapine (leponex) [Ergebnisse europäischer multicenter-doppelblindstudien mit clozapin (Leponex)]. In: Berner P, editors(s). Clozapin: Zweites Symposium. Wien: Pharmazeutika Wander, Biochemie GesmbH, 1976:8-19.
-
- Fischer-Cornelssen KA, Ferner UJ. An example of European multicenter trials: multispectral analysis of clozapine. Psychopharmacology Bulletin 1976;12:34-9. - PubMed
Gan 2017 {published data only}
-
- Gan JL, Duan HF, Cheng ZX, Yang JM, Zhu XQ, Gao CY, et al. Neuroprotective effect of modified electroconvulsive therapy for schizophrenia: a proton magnetic resonance spectroscopy study. Journal of Nervous and Mental Disease 2017;205(6):480-6. - PubMed
Gerlach 1974 {published data only}
-
- Gerlach J, Koppelhus P, Helweg E, Monrad A. Clozapine and haloperidol in a single-blind cross-over trial: therapeutic and biochemical aspects in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 1974;50(4):410-24. - PubMed
-
- Gerlach J, Munkvad I. Clozapin og haloperidol: Kliniske og biokemiske aspekter i skizofrenibehandlingen. Nordisk Psykiatrisk Tidsskrift 1974;28(6):463.
Gilgash 1957 {published data only}
-
- Gilgash CA. Effects of thorazine on Wechsler scores of adult catatonic schizophrenics. Psychological Reports 1957;3:561-4.
-
- Gilgash CA. Thorazine therapy with catatonic schizophrenics in relation to Wechsler verbal and performance subtest comparison. Journal of Clinical Psychology 1961;17(1):95. - PubMed
Liu 2008 {published data only (unpublished sought but not used)}
-
- Liu T, Wang S. A comparative study of modified electroconvulsive therapy for catatonic schizophrenia [Wuchouchu Dianjingluan Zhiliao Jinzhangxing Jingshen Fenliezheng de Duizhao Yanjiu]. Shandong Jingshen Yixue [Shandong Archives of Psychiatry] 2008;21(1):32-4.
Mori 1989 {published data only}
-
- Mori A, Kazamatsuri H, Kaneno S, Kamijima K, Kariya T, Murasaki M, et al. A double blind comparison of a new benzamide compound YM 09151 with haloperidol in the treatment of schizophrenia. Rinsho Hyoka 1989;17(3-4):349-77.
-
- Mori A, Kazamatsuri H, Kaneno S, Kamijima K, Kariya T, Murasaki M, et al. A double-blind comparison of emonapride with haloperidol in the treatment of schizophrenia. In: 17th Collegium Internationale Neuro-Psychopharmacologicum Congress; 1990 Sep 10-14; Kyoto, Japan. 1990:296.
NCT00034905 {published data only}
-
- AstraZeneca. A multicenter, double-blind, randomized comparison of the efficacy and safety of quetiapine fumarate (Seroquel) and risperidone (Risperdal) in the treatment of patients with schizophrenia. (This study was accessed for the Cochrane database on 10 March 2008 from a web-based repository of pharmaceutical industry clinical trials (www.clinicalstudyresults.org/), which was established by the Pharmaceutical Research and Manufacturers of America but was phased out 20 December 2011.) (first received 7 December 2018).
-
- NCT00034905. A comparison of Seroquel vs. Risperidone in schizophrenia. clinicaltrials.gov/ct2/show/NCT00034905 (first received 7 December 2018).
NCT00204061 {published data only}
-
- NCT00204061. Early pharmacological and psychological intervention for late prodromal states of psychosis. clinicaltrials.gov/ct2/show/NCT00204061 (first received 7 December 2018).
NCT00210717 {published data only}
-
- NCT00210717. A randomized, double-blind, parallel group, comparative study of flexibly dosed paliperidone palmitate (25, 50, 75, or 100 mg eq.) administered every 4 weeks and flexibly dosed risperdal consta (25, 37.5, or 50 mg) administered every 2 weeks in subjects with schizophrenia. clinicaltrials.gov/ct2/show/NCT00210717 (first received 7 December 2018).
NCT00505076 {published data only}
-
- NCT00505076. MK-0777 for the treatment of cognitive impairments in patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT00505076 (first received 7 December 2018).
NCT00520923 {published data only}
-
- Eli Lilly and Company. A study for patients with schizophrenia. Eli Lilly and Company Clinical Trial Registry (This reference is for a study listed in the proprietary Eli Lilly Clinical Trial Registry. It was also listed secondarily and is available publicly at clinicaltrials.gov/ct2/show/NCT00520923.) (first received 7 December 2018).
-
- Kinon B. mGlu2/3 receptor agonists – a non D2 approach to treating schizophrenia. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):53.
-
- Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biological Psychiatry 2015;78(11):754-62. - PubMed
-
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of ly2140023 monohydrate in patients with DSM-IV schizophrenia. Journal of Clinical Psychopharmacology 2011;31(3):349-55. - PubMed
-
- Kinon BJ, Zhang L, Williams JE, Osuntokun OO, Millen BA, Kollack-Walker S. Ly2140023 monohydrate: an agonist at the mglu2/3 receptor for the treatment of schizophrenia. Schizophrenia Research 2010;117(2-3):379.
Sarai 1987 {published data only}
-
- Sarai K, Okada M. Comparison of efficacy of zotepine and thiothixene in schizophrenia in a double-blind study. Pharmacopsychiatry 1987;20(1):38-46. - PubMed
Smith 1961 {published data only}
-
- Smith K, Moody AC. The effect of meprobamate (miltown), RO 1-9569-12 (nitoman), and SCH-6673 (tindal) on the odour of schizophrenic sweat. American Journal of Psychiatry 1961;118:1034-5.
Tegeler 1979 {published data only}
-
- Tegeler J, Floru L. A comparative study of the longacting neuroleptics perphenazin enanthate and fluspirilene [Eine Vergleichende Untersuchung Der Depot-Neuroleptika Perphenazin Onanthat Und Fluspirilen]. Pharmakopsychiatrie Und Neuropsychopharmakologie 1979;12(5):357-65. - PubMed
Wang 2012 {published data only}
-
- Wang Y, Liu H, Xia J. Comparative study of modified ECT treatment for schizophrenia with stuporous state [Wuchouchu Dianxiuke Zhiliao Jingshen Fenliezheng Mujiang Xingwei Duizhao Yanjiu]. Dangdai Yixue [China Contemporary Medicine] 2012;18(16):79-80.
Wang 2016 {published data only}
-
- Wang J. Clinical analysis of low-dose amisulpride in the treatment of stupor in schizophrenia [Xiao Jiliang Anhuangbili Zhiliao Jingshen Fenliezheng Mujiang Zhuangtai Linchuang Fenxi]. Zhongguo Shiyong Shenjing Jibing Zazhi [Chinese Journal of Practical Nervous Diseases] 2016;19(19):56-8.
Yu 2005 {published data only}
-
- Yu DS, Xu J, Sun DH, Zeng YY, Gao XN, Jiang XJ. A comparative study of clozapine vs risperidone on body posture balance. Zhongguo Xinyao Yu Linchuang Zazhi [Chinese Journal of New Drugs and Clinical Remedies] 2005;24(2):125-8.
Additional references
Abrams 1976
-
- Abrams R, Taylor MA. Catatonia: a prospective clinical study. Archives of General Psychiatry 1976;33(5):579-81. - PubMed
Altman 1996
American Psychiatric Association 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Publishing, 2013.
Ananth 2004
-
- Ananth J, Parameswaran S, Gunatilake S, Burgoyne K, Sidhom T. Neuroleptic malignant syndrome and atypical antipsychotic drugs. Journal of Clinical Psychiatry 2004;65(4):464-70. - PubMed
Andreasen 1983
-
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City (IA): University of Iowa, 1983.
Arora 2017
-
- Arora M, Banal R, Praharaj SK, Mahajn V. Amisulpride augmentation in acute catatonia. American Journal of Therapeutics 2017;24:e381–5. - PubMed
Astrup 1964
-
- Astrup C, Fish F. The response of the different Leonhard subgroups of schizophrenia of psychotropic drugs. Folia Psychiatrica et Neurologica 1964;18:133-40. - PubMed
Beach 2017
-
- Beach SR, Gomez-Bernal F, Huffman JC, Fricchione GL. Alternative treatment strategies for catatonia: a systematic review. General Hospital Psychiatry 2017;48:1-19. - PubMed
Beckmann 1992
-
- Beckmann H, Fritze J, Franzek E. The influence of neuroleptics on specific syndromes and symptoms in schizophrenics with unfavorable long-term course. Neuropsychobiology 1992;26:50-8. - PubMed
Berardi 2002
-
- Berardi D, Dell'Atti M, Amore M, De Ronchi D, Ferrari G. Clinical risk factors for neuroleptic malignant syndrome. Human Psychopharmacology 2002;17(2):99-102. - PubMed
Bland 1997
Blumer 1997
-
- Blumer D. Catatonia and the neuroleptics: psychobiologic significance of remote and recent findings. Comprehensive Psychiatry 1997;38(4):193-201. - PubMed
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Aperçu sur la problématique des indices d'efficacité thérapeutique. 3. Comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405-11. [PMID: ] - PubMed
Buchanan 2011
Bush 1996
-
- Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatrica Scandinavica 1996;93:129-36. - PubMed
Carbon 2018
Caroff 2002
-
- Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. Journal of Clinical Psychiatry 2002;63(Suppl 4):12-9. - PubMed
Caroff 2004
-
- Caroff SN, Mann SC, Campbell EC, Sullivan KA. Epidemiology. In: Caroff SN, Mann SC, Francis A, Fricchione GL, editors(s). Catatonia: From Psychopathology to Neurobiology. Washington, DC: American Psychiatric Publishing, 2004:15-32.
Caroff 2011
Caroff 2021
Carpenter 1994
-
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681-90. - PubMed
Carroll 2006
-
- Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Annals of Clinical Psychiatry 2006;18(2):133-4. - PubMed
Ceskova 1993
-
- Ceskova E, Svestka J. Double-blind comparison of risperidone and haloperidol in schizophrenic and schizoaffective psychoses. Pharmacopsychiatry 1993;26:121-4. - PubMed
Chattopadhyay 2012
Christancho 2013
-
- Cristancho MA, Cristancho PO, Reardon JP. Other therapeutic psychiatric uses of superficial brain stimulation. Handbook of Clinical Neurology 2013;116:415-22. - PubMed
Clinebell 2014
-
- Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. Journal of Clinical Psychiatry 2014;75(6):644-51. - PubMed
Compton 2015
Cuesta 2016
-
- Cuesta MJ, Peralta V. Going beyond classic descriptions to future phenomenology of schizophrenia. JAMA Psychiatry 2016;73:1010-2. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta-analyses of binary data. In: 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623-9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971-80. - PubMed
Dursun 2005
-
- Dursun SM, Hallak JEC, Haddad P, Leahy A, Byrne A, Strickland PL, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? Journal of Psychopharmacology 2005;19:432-3. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Fink 2001
-
- Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. Journal of Neural Transmission (Vienna, Austria: 1996) 2001;108(6):637-44. - PubMed
Fink 2003
-
- Fink M, Taylor MA. Catatonia: A Clinician's Guide to Diagnosis and Treatment. Cambridge: Cambridge University Press, 2003.
Fleischhacker 2012
-
- Fleischhacker WW, Gopal S, Lane R, Gassmann-Mayer C, Lim P, Hough D, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. International Journal of Neuropsychopharmacology 2012;15:107-18. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. - PubMed
Gazdag 2009
-
- Gazdag G, Mann SC, Ungavri GS, Caroff SN. Clinical evidence for the efficacy of electroconvulsive therapy in the treatment of catatonia and psychoses. In: Swartz CM, editors(s). Electroconvulsive and Neuromodulation Therapies. Cambridge: Cambridge University Press, 2009:124-48.
Gelenberg 1977
-
- Gelenberg AJ, Mandel MR. Catatonic reactions to high-potency neuroleptic drugs. Archives of General Psychiatry 1977;34:947-50. - PubMed
Gibson 2008
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876-83. - PubMed
Hatta 2007
-
- Hatta K, Miyakawa K, Ota T, Usui C, Nakamura H, Arai H. Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. Journal of Electroconvulsive Therapy 2007;23:233-5. - PubMed
Hawkins 1995
-
- Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatment of catatonia. International Journal of Psychiatry in Medicine 1995;25(4):345-69. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2020
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available at training.cochrane.org/handbook/archive/v6.1.
Honigfeld 1965
-
- Honigfeld G, Gillis RD, Klett CJ. Nurses' observation scale for inpatient evaluation: a new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65-71. - PubMed
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27-30. [PMID: ] - PubMed
Katzung 2015
-
- Katzung BG, Trevor AJ. Basic and Clinical Pharmacology. New York: McGraw-Hill Education, 2015.
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi-Health Systems, 1986.
Kendler 2016
-
- Kendler KS. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiatry 2016;73(10):1082-92. - PubMed
King 1998
-
- King DJ, Link CG, Kowalcyk B. A comparison of bd and tid dose regimens of quetiapine (Seroquel) in the treatment of schizophrenia. Psychopharmacology 1998;137:139-46. - PubMed
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M, et al. Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley and Sons, 2019:67-107.
Leon 2006
-
- Leon AC, Mallinckrodt CH, Chuang-Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001-5. [PMID: ] - PubMed
Leonhard 1999
-
- Leonhard K. Classification of Endogenous Psychoses and their Differential Etiology. 2nd edition. Wien: Springer Verlag, 1999.
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research 2005;79(2-3):231-8. [PMID: ] - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366-71. [PMID: ] - PubMed
Leucht 2013
-
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951-62. - PubMed
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209-23. - PubMed
Lopez‐Canino 2004
-
- Lopez-Canino A, Francis A. Drug-induced catatonia. In: Caroff SN, Mann SC, Francis A, Fricchione GL, editors(s). Catatonia: From Psychopathology to Neurobiology. Washington, DC: American Psychiatric Publishing, 2004:129-39.
Lynch 2001
-
- Lynch J, Morrison J, Graves N, Meddis D, Drummond MF, Hellewell JSE. The health economic implications of treatment with quetiapine: an audit of long-term treatment for patients with chronic schizophrenia. European Psychiatry 2001;16:307-12. - PubMed
Mann 1986
-
- Mann SC, Caroff SN, Bleier HR, Welz WK, Kling MA, Hayashida M. Lethal catatonia. American Journal of Psychiatry 1986;143(11):1374-81. - PubMed
Mann 2003
-
- Mann SC, Caroff SN, Keck PE, Lazarus A. Neuroleptic Malignant Syndrome and Related Conditions. Washington, DC: American Psychiatric Publishing, 2003.
Manschreck 1982
-
- Manschreck TC, Maher BA, Rucklos ME, Vereen DR. Disturbed voluntary motor activity in schizophrenic disorder. Psychological Medicine 1982;12:73-84. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. - PubMed
Martenyi 2001
-
- Martenyi F, Metcalfe S, Schausberger B, Dossenbach MR. An efficacy analysis of olanzapine treatment data in schizophrenia patients with catatonic signs and symptoms. Journal of Clinical Psychiatry 2001;62 Suppl 2:25-7. - PubMed
Matar 2014
McKenna 1991
-
- McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia. 2: The 'conflict of paradigms' hypothesis. British Journal of Psychiatry 1991;158:328-36. - PubMed
Miller 1953
-
- Miller DH, Clancy J, Cumming E. A comparison between unidirectional current nonconvulsive electrical stimulation given with Reiter's machine, standard alternating current electro-shock (Cerletti method), and pentothal in chronic schizophrenia. American Journal of Psychiatry 1953;109(8):617-20. - PubMed
Morozova 2014
-
- Morozova MA, Rupchev GE, Kozhekin IG, Arsenieva TB, Cheremin RA, Yachmenev VN, et al. The use of acatinol in the treatment of stable patients with schizophrenia with predominance of behaviour disorganization and subcatatonic features. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2014;114(1):35-41. - PubMed
Morrens 2015
-
- Morrens M, Docx L, Sabbe B. Catatonia as part of the psychomotor syndrome in schizophrenia. Future Neurology 2015;10:423-9.
Naber 1992
-
- Naber D, Holzbach R, Perro C, Hippius H. Clinical management of clozapine patients in relation to efficacy and side-effects. British Journal of Psychiatry 1992;17(Suppl):54-9. - PubMed
Narayanaswamy 2012
-
- Narayanaswamy JC, Tibrewal P, Zutshi A, Srinivasaraju R, Math SB. Clinical predictors of response to treatment in catatonia. General Hospital Psychiatry 2012;34(3):312-6. - PubMed
Osman 1994
-
- Osman AA, Khurasani MH. Lethal catatonia and neuroleptic malignant syndrome. A dopamine receptor shut-down hypothesis. British Journal of Psychiatry 1994;165(4):548-50. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799-812.
Panteleeva 1987
-
- Panteleeva GP, Kovskaya MY, Belyaev BS, Minsker EI, Vynar O, Ceskova E, et al. Clozapine in the treatment of schizophrenia patients: an international multicenter trial. Clinical Therapeutics 1987;10(1):57-68. - PubMed
Pataki 1992
-
- Pataki J, Zervas IM, Jandorf L. Catatonia in a university inpatient service (1985-1990). Convulsive Therapy 1992;8(3):163-73. - PubMed
Peralta 1999
-
- Peralta V, Cuesta MJ. Negative parkinsonian, depressive and catatonic symptoms in schizophrenia: a conflict of paradigms revisited. Schizophrenia Research 1999;40(3):245-53. - PubMed
Peralta 2001
-
- Peralta V, Cuesta MJ. Motor features in psychotic disorders. II. Development of diagnostic criteria for catatonia. Schizophrenia Research 2001;47(2-3):117-26. - PubMed
Peralta 2010
-
- Peralta V, Campos MS, Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophrenia Research 2010;118(1-3):168-75. - PubMed
Philbrick 1994
-
- Philbrick KL, Rummans TA. Malignant catatonia. Journal of Neuropsychiatry and Clinical Neurosciences 1994;6(1):1-13. - PubMed
Phutane 2011
Roberts 2021
Rohland 1993
-
- Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. Journal of Affective Disorders 1993;29(4):255-61. - PubMed
Rosebush 1999
-
- Rosebush PI, Mazurek MF. Catatonia: re-awakening to a forgotten disorder. Movement Disorders 1999;14(3):395-7. - PubMed
Sackeim 1987
-
- Sackeim HA, Ross GR, Hopkins N. Subjective side effects acutely following ECT: association of treatment modality and clinical response. Convulsive Therapy 1987;2:100-10. - PubMed
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available at training.cochrane.org/handbook/archive/v6.1.
Shokraneh 2017
Shokraneh 2019
Shokraneh 2020
-
- Shokraneh F, Adams CE. Cochrane Schizophrenia Group’s Study-Based Register of Randomized Controlled Trials: Development and Content Analysis. Schizophrenia Bulletin Open 2020;1(1):sgaa061.
Shokraneh 2021
Sienaert 2014
Simpson 1970
-
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. Supplementum 1970;212:11-19. - PubMed
Sinclair 2014
Sinclair 2019
Sterne 2019
-
- Sterne JAC, Savović J, Page MJ, Elbers RGE, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:I4898. - PubMed
Stubner 2004
-
- Stubner S, Rustenbeck E, Grohmann R, Wagner G, Engel R, Neundorfer G, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry 2004;37(Suppl 1):S54-64. - PubMed
Svestka 1990
-
- Svestka J, Ceskova E, Rysanek R, Obrovska V. Double-blind clinical comparison of risperidon and haloperidol in acute schizophrenic and schizoaffective psychoses. Activitas Nervosa Superior 1990;32:237-38.
Tabbane 2016
Taylor 2018
-
- Taylor D, Barnes TR, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th edition. Hoboken, NJ: Wiley Blackwell, 2018.
Trikalinos 2004
-
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57:1124-30. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii-92. - PubMed
Ungvari 1999
-
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology 1999;142(4):393-8. - PubMed
Ungvari 2001
-
- Ungvari GS, Kau LS, Wai-Kwong T, Shing NF. The pharmacological treatment of catatonia: an overview. European Archives of Psychiatry and Clinical Neuroscience 2001;251(Suppl 1):131-4. - PubMed
Ungvari 2005
-
- Ungvari GS, Leung SK, Ng FS, Cheung HK, Leung T. Schizophrenia with prominent catatonic features ('catatonic schizophrenia'): I. Demographic and clinical correlates in the chronic phase. Progress in Neuropsychopharmacology & Biological Psychiatry 2005;29(1):27-38. - PubMed
Ungvari 2010a
-
- Ungvari GS. Amineptine treatment of persistent catatonic symptoms in schizophrenia: a controlled study. Neuropsychopharmacologia Hungarica 2010;12(4):463-7. - PubMed
Ungvari 2010b
Ungvari 2018
-
- Ungvari GS, Gerevich J, Takacs R, Gazdag G. Schizophrenia with prominent catatonic features: a selective review. Schizophrenia Research 2018;200:77-84. - PubMed
Van Den Eede 2005
-
- Van Den Eede F, Van Hecke J, Van Dalfsen A, Van den Bossche B, Cosyns P, Sabbe BG. The use of atypical antipsychotics in the treatment of catatonia. European Psychiatry 2005;20(5-6):422-9. - PubMed
Weiden 2016
White 1991
-
- White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. British Journal of Psychiatry 1991;158:419-21. - PubMed
White 2000
-
- White DA, Robins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):58-65. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous