Phase II proof-of-concept study of atorvastatin in castration-resistant prostate cancer
- PMID: 35844167
- PMCID: PMC10087532
- DOI: 10.1111/bju.15851
Phase II proof-of-concept study of atorvastatin in castration-resistant prostate cancer
Abstract
Objectives: To test for evidence of statin-mediated effects in patients with castration-resistant prostate cancer (CRPC) as post-diagnosis use of statins in patients with prostate cancer is associated with favourable survival outcome.
Patients and methods: The SPECTRE trial was a 6-weeks-long proof-of-concept single-arm Phase II treatment trial, combining atorvastatin and androgen deprivation therapy in patients with CRPC (regardless of metastatic status), designed to test for evidence of statin-mediated effects in patients with CRPC. The primary study endpoint was the proportion of patients achieving a ≥50% drop from baseline in prostate-specific antigen (PSA) levels at any time over the 6-week period of atorvastatin medication (PSA response). Exploratory endpoints include PSA velocity and serum metabolites identified by mass spectrometry .
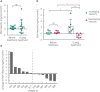
Results: At the scheduled interim analysis, one of 12 patients experienced a ≥50% drop in PSA levels (primary endpoint), with ≥2 patients satisfying the primary endpoint required for further recruitment. All 12 patients experienced substantial falls in serum cholesterol levels following statin treatment. While all patients had comparable pre-study PSA velocities, six of 12 patients showed decreased PSA velocities after statin treatment, suggestive of disease stabilization. Unbiased metabolomics analysis on serial weekly blood samples identified tryptophan to be the dominant metabolite associated with patient response to statin.
Conclusions: Data from the SPECTRE study provide the first evidence of statin-mediated effects on CRPC and early sign of disease stabilization. Our data also highlight the possibility of altered tryptophan metabolism being associated with tumour response.
Keywords: #PCSM; #ProstateCancer; #uroonc; atorvastatin; castration resistant prostate cancer; cholesterol; prostate specific antigen; statins.
© 2022 The Authors. BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30 - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration‐resistant prostate cancer progressing after docetaxel treatment: a randomised open‐label trial. Lancet 2010; 376: 1147–54 - PubMed
-
- Davis ID, Martin AJ, Stockler MR et al. Enzalutamide with standard first‐line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

