Stimulant and non-stimulant drug therapy for people with attention deficit hyperactivity disorder and epilepsy
- PMID: 35844168
- PMCID: PMC9289704
- DOI: 10.1002/14651858.CD013136.pub2
Stimulant and non-stimulant drug therapy for people with attention deficit hyperactivity disorder and epilepsy
Abstract
Background: Attention Deficit Hyperactivity Disorder (ADHD) can co-occur in up to 40% of people with epilepsy. There is debate about the efficacy and tolerability of stimulant and non-stimulant drugs used to treat people with ADHD and co-occurring epilepsy.
Objectives: To assess the effect of stimulant and non-stimulant drugs on children and adults with ADHD and co-occurring epilepsy in terms of seizure frequency and drug withdrawal rates (primary objectives), as well as seizure severity, ADHD symptoms, cognitive state, general behaviour, quality of life, and adverse effects profile (secondary objectives).
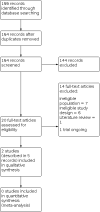
Search methods: We searched the following databases on 12 October 2020: Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to 9 October 2020), CINAHL Plus (EBSCOhost, 1937 onwards). There were no language restrictions. CRS Web includes randomised or quasi-randomised controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialised Registers of Cochrane Review Groups including Epilepsy. SELECTION CRITERIA: We included randomised controlled trials of stimulant and non-stimulant drugs for people of any age, gender or ethnicity with ADHD and co-occurring epilepsy.
Data collection and analysis: We selected articles and extracted data according to predefined criteria. We conducted primary analysis on an intention-to-treat basis. We presented outcomes as risk ratios (RRs) with 95% confidence intervals (CIs), except for individual adverse effects where we quoted 99% CIs. We conducted best- and worst-case sensitivity analyses to deal with missing data. We carried out a risk of bias assessment for each included study using the Cochrane risk of bias tool and assessed the overall certainty of evidence using the GRADE approach.
Main results: We identified two studies that matched our inclusion criteria: a USA study compared different doses of the stimulant drug osmotic-release oral system methylphenidate (OROS-MPH) with a placebo in 33 children (mean age 10.5 ± 3.0 years), and an Iranian study compared the non-stimulant drug omega-3 taken in conjunction with risperidone and usual anti-seizure medication (ASM) with risperidone and ASM only in 61 children (mean age 9.24 ± 0.15 years). All children were diagnosed with epilepsy and ADHD according to International League Against Epilepsy and Diagnostic and Statistical Manual of Mental Disorders, fourth edition, criteria, respectively. We assessed both studies to be at low risk of detection and reporting biases, but assessments varied from low to high risk of bias for all other domains. OROS-MPH No participant taking OROS-MPH experienced significant worsening of epilepsy, defined as: 1. a doubling of the highest 14-day or highest two-day seizure rate observed during the 12 months before the trial; 2. a generalised tonic-clonic seizure if none had been experienced in the previous two years; or 3. a clinically meaningful intensification in seizure duration or severity (33 participants, 1 study; low-certainty evidence). However, higher doses of OROS-MPH predicted an increased daily risk of a seizure (P < 0.001; 33 participants, 1 study; low-certainty evidence). OROS-MPH had a larger proportion of participants receiving 'much improved' or 'very much improved' scores for ADHD symptoms on the Clinical Global Impressions for ADHD-Improvement tool (33 participants, 1 study; low-certainty evidence). OROS-MPH also had a larger proportion of people withdrawing from treatment (RR 2.80; 95% CI 1.14 to 6.89; 33 participants, 1 study; moderate-certainty evidence). Omega-3 Omega-3 with risperidone and ASM were associated with a reduction in mean seizure frequency by 6.6 seizures per month (95% CI 4.24 to 8.96; 56 participants, 1 study; low-certainty evidence) and an increase in the proportion of people achieving 50% or greater reduction in monthly seizure frequency (RR 2.79, 95% CI 0.84 to 9.24; 56 participants, 1 study; low-certainty evidence) compared to people on risperidone and ASM alone. Omega-3 with risperidone and ASM also had a smaller proportion of people withdrawing from treatment (RR 0.65, 95% CI 0.12 to 3.59; 61 participants, 1 study; low-certainty evidence) but a larger proportion of people experiencing adverse drug events (RR 1.40, 95% CI 0.44 to 4.42; 56 participants, 1 study; low-certainty evidence) compared to people on risperidone and ASM alone.
Authors' conclusions: In children with a dual-diagnosis of epilepsy and ADHD, there is some evidence that use of the stimulant drug OROS-MPH is not associated with significant worsening of epilepsy, but higher doses of it may be associated with increased daily risk of seizures; the evidence is of low-certainty. OROS-MPH is also associated with improvement in ADHD symptoms. However, this treatment was also associated with a large proportion of treatment withdrawal compared to placebo. In relation to the non-stimulant drug omega-3, there is some evidence for reduction in seizure frequency in children who are also on risperidone and ASM, compared to children who are on risperidone and ASM alone. Evidence is inconclusive whether omega-3 increases or decreases the risk of adverse drug events. We identified only two studies - one each for OROS-MPH and omega-3 - with low to high risk of bias. We assessed the overall certainty of evidence for the outcomes of both OROS-MPH and omega-3 as low to moderate. More studies are needed. Future studies should include: 1. adult participants; 2. a wider variety of stimulant and non-stimulant drugs, such as amphetamines and atomoxetine, respectively; and 3. additional important outcomes, such as seizure-related hospitalisations and quality of life. Clusters of studies which assess the same drug - and those that build upon the evidence base presented in this review on OROS-MPH and omega-3 - are needed to allow for meta-analysis of outcomes.
Antecedentes: El trastorno por déficit de atención e hiperactividad (TDAH) puede concurrir en hasta el 40% de las personas con epilepsia. Existe un debate sobre la eficacia y la tolerabilidad de los fármacos estimulantes y no estimulantes utilizados para tratar a las personas con TDAH y epilepsia concurrente.
Objetivos: Evaluar el efecto de los fármacos estimulantes y no estimulantes en niños y adultos con TDAH y epilepsia concurrente, en cuanto a la frecuencia de las crisis epilépticas y las tasas de retiro del fármaco (objetivos principales), así como la gravedad de las crisis epilépticas, los síntomas del TDAH, el estado cognitivo, el comportamiento general, la calidad de vida y el perfil de efectos adversos (objetivos secundarios). MÉTODOS DE BÚSQUEDA: El 12 de octubre de 2020 se realizaron búsquedas en las siguientes bases de datos: Registro Cochrane de Estudios (CRS Web), MEDLINE (Ovid, 1946 hasta el 9 de octubre de 2020), CINAHL Plus (EBSCOhost, 1937 en adelante). No hubo restricciones de idioma. El CRS Web incluye ensayos controlados aleatorizados o cuasialeatorizados de PubMed, Embase, ClinicalTrials.gov, la Plataforma de registros internacionales de ensayos clínicos (ICTRP) de la Organización Mundial de la Salud, el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials; CENTRAL) y los registros especializados de los Grupos Cochrane de Revisión, incluido el de Epilepsia. CRITERIOS DE SELECCIÓN: Se incluyeron ensayos controlados aleatorizados de fármacos estimulantes y no estimulantes para personas de cualquier edad, sexo o etnia con TDAH y epilepsia concurrente. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Se seleccionaron los artículos y se extrajeron los datos según criterios predefinidos. El análisis principal se realizó por intención de tratar. Los desenlaces se presentaron como razones de riesgos (RR) con intervalos de confianza (IC) del 95%, excepto en el caso de los efectos adversos individuales, en los que se citaron los IC del 99%. Se realizaron análisis de sensibilidad en el mejor y peor de los casos para lidiar con los datos faltantes. Se realizó una evaluación del riesgo de sesgo para cada estudio incluido mediante la herramienta Cochrane de riesgo de sesgo y la certeza general de la evidencia se evaluó mediante el método GRADE.
Resultados principales: Se identificaron dos estudios que cumplieron con los criterios de inclusión: un estudio de EE.UU. comparó diferentes dosis del fármaco estimulante metilfenidato con un sistema oral de liberación osmótica (OROS‐MPH) con un placebo en 33 niños (media de edad 10,5 ± 3,0 años), y un estudio iraní comparó el fármaco no estimulante omega‐3 tomado junto con la risperidona y la medicación anticonvulsiva (MAC) habitual con la risperidona y la MAH solamente en 61 niños (media de edad 9,24 ± 0,15 años). Todos los niños tenían un diagnóstico de epilepsia y TDAH según los criterios de la International League Against Epilepsy y del Diagnostic and Statistical Manual of Mental Disorders, cuarta edición, respectivamente. Se consideró que ambos estudios tenían un riesgo de sesgo de detección y de notificación bajos, pero las evaluaciones variaron de riesgo de sesgo bajo a alto en todos los demás dominios. OROS‐MPH Ningún participante de los que recibieron OROS‐MPH presentó un empeoramiento significativo de la epilepsia, definido como: 1. una duplicación de la tasa más alta de convulsiones en 14 días o en dos días, observada durante los 12 meses anteriores al ensayo; 2. una convulsión tónico‐clónica generalizada si no se había experimentado ninguna en los dos años anteriores; o 3. una intensificación clínicamente significativa de la duración o la gravedad de las convulsiones (33 participantes, un estudio; evidencia de certeza baja). Sin embargo, las dosis más altas de OROS‐MPH predijeron un mayor riesgo diario de presentar una convulsión (p < 0,001; 33 participantes, un estudio; evidencia de certeza baja). Con el OROS‐MPH hubo una mayor proporción de participantes que recibieron puntuaciones de "mucha mejoría" o "muchísima mejoría" en los síntomas del TDAH según la herramienta Clinical Global Impressions for ADHD‐Improvement (33 participantes, un estudio; evidencia de certeza baja). Con el OROS‐MPH también hubo una mayor proporción de personas que se retiraron del tratamiento (RR 2,80; IC del 95%: 1,14 a 6,89; 33 participantes, un estudio; evidencia de certeza moderada). Omega‐3 El omega‐3 con la risperidona y la MAC se asociaron con una reducción de la frecuencia media de las crisis epilépticas en 6,6 crisis epilépticas por mes (IC del 95%: 4,24 a 8,96; 56 participantes, un estudio; evidencia de certeza baja) y un aumento de la proporción de personas que lograron una reducción del 50% o más en la frecuencia mensual de las crisis epilépticas (RR: 2,79; IC del 95%: 0,84 a 9,24; 56 participantes, un estudio; evidencia de certeza baja) en comparación con las personas que recibieron risperidona y MAC solamente. Con el omega‐3 con risperidona y MAC también hubo una menor proporción de personas que se retiraron del tratamiento (RR 0,65; IC del 95%: 0,12 a 3,59; 61 participantes, un estudio; evidencia de certeza baja), pero una mayor proporción de personas que presentaron eventos adversos al fármaco (RR 1,40; IC del 95%: 0,44 a 4,42; 56 participantes, un estudio; evidencia de certeza baja) en comparación con las personas que recibieron risperidona y MAC solamente.
Conclusiones de los autores: En los niños con un doble diagnóstico de epilepsia y TDAH, hay alguna evidencia de que el uso del fármaco estimulante OROS‐MPH no se asocia con un empeoramiento significativo de la epilepsia, pero las dosis más altas podrían estar asociadas con un mayor riesgo diario de crisis epilépticas; la evidencia es de certeza baja. OROS‐MPH también se asocia con una mejoría de los síntomas del TDAH. Sin embargo, este tratamiento también se asoció con una gran proporción de retiro del tratamiento en comparación con el placebo. En relación con el fármaco no estimulante omega‐3, existe alguna evidencia de una reducción de la frecuencia de las convulsiones en los niños que también recibieron risperidona y MAC, en comparación con los niños que sólo recibieron risperidona y MAC. La evidencia no es concluyentes en cuanto a si los omega‐3 aumentan o disminuyen el riesgo de experimentar efectos adversos de los medicamentos. Sólo se identificaron dos estudios (uno con OROS‐MPH y otro con omega‐3) con un riesgo de sesgo bajo a alto. La certeza general de la evidencia para los desenlaces de OROS‐MPH y omega‐3 se consideró baja a moderada. Se necesitan más estudios. Los estudios futuros deberían incluir: 1. participantes adultos; 2. una mayor variedad de fármacos estimulantes y no estimulantes, como las anfetaminas y la atomoxetina, respectivamente; y 3. desenlaces adicionales importantes, como las hospitalizaciones relacionadas con las convulsiones y la calidad de vida. Se necesitan grupos de estudios que evalúen el mismo fármaco (y estén desarrollados sobre evidencia presentada en esta revisión acerca de OROS‐MPH y omega‐3) para poder realizar un metanálisis de los desenlaces.
Trial registration: ClinicalTrials.gov NCT00323947.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
CE: none to declare
KY: none to declare
VW: none to declare
GM: none to declare
SR: none to declare
RC: has provided consultancy for Eisai, GW Pharma, Zogenix. He has received payment for lectures from GW Pharma, Zogenix and has received support for attendance to a conference to present results of a separate Eisai sponsored study. RC has shares in RIZE Medical Cannabis and Life Sciences. RC monies are paid to his institution.
Figures










Update of
- doi: 10.1002/14651858.CD013136
References
References to studies included in this review
Fallah 2018 {published data only}
Gonzalez‐Heydrich 2010 {published data only}
-
- Gonzalez-Heydrich J. OROS methylphenidate for attention-deficit/hyperactivity disorder plus epilepsy. P and T (Pharmacy and Therapeutics) 2006;31(12):725-6.
References to studies excluded from this review
Adams 2017 {published data only}
-
- Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, et al. Methylphenidate, cognition, and epilepsy: a 1-month open-label trial. Epilepsia 2017;58(12):2124-32. [PMID: ] - PubMed
Dave 1993 {published data only}
-
- Dave UP, Chauvan V, Dalvi J. Evaluation of BR-16 A (Mentat) in cognitive and behavioural dysfunction of mentally retarded children - a placebo-controlled study. Indian Journal of Pediatrics 1993;60(3):423-8. [PMID: ] - PubMed
Eapen 2005 {published data only}
-
- Eapen V, Gururaj A. An open clinical trial of risperidone in children with seizures and hyperactivity. Journal of Pediatric Neurology 2005;3(1):31-4. [DOI: 10.1055/s-0035-1557234] - DOI
Feldman 1989 {published data only}
-
- Feldman H, Crumrine P, Handen BL, Alvin R, Teodori J. Methylphenidate in children with seizures and attention-deficit disorder. American Journal of Diseases of Children 1989;143(9):1081-6. [PMID: ] - PubMed
Frank 1993 {published data only}
-
- Frank Y. Visual event related potentials after methylphenidate and sodium valproate in children with attention deficit hyperactivity disorder. Clinical Electroencephalography 1993;24(1):19-24. [PMID: ] - PubMed
Ghose 1983 {published data only}
-
- Ghose K. l-Tryptophan in hyperactive child syndrome associated with epilepsy: a controlled study. Neuropsychobiology 1983;10(2-3):111-4. [PMID: ] - PubMed
Gross‐Tsur 1997 {published data only}
-
- Gross-Tsur V, Manor O, Meere J, Joseph A, Shalev RS. Epilepsy and attention deficit hyperactivity disorder: is methylphenidate safe and effective? [Republished from Journal of Pediatrics; 1997 Jan;130(1):40-4; PMID: 9003849]. Journal of Pediatrics 1997;130(4):670-4. [PMID: ] - PubMed
Gunning 2004 {published data only}
-
- Gunning WB. The efficacy of methylphenidate in children with epilepsy and ADHD: the role of dosage, epilepsy type and psychiatric comorbidity. Epilepsia 2004;45(Suppl 3):206. [Abstract no: p605]
Ikeda 1996 {published data only}
-
- Ikeda A, Shibasaki H, Tashiro K, Mizuno Y, Kimura J. Clinical trial of piracetam in patients with myoclonus: nationwide multiinstitution study in Japan. The Myoclonus/Piracetam Study Group. Movement Disorders 1996;11(6):691-700. [PMID: ] - PubMed
Moorthy 2018 {published data only}
-
- Moorthy MP, Srinivasan AV, Bhanu K, Mugundan K, Sivakumar S. L-Carnosine in pediatric cognitive disorders. Neurorehabilitation and Neural Repair 2018;32(4-5):372-3. [DOI: 10.1177/1545968318765498] - DOI
Swanson 2008 {published data only}
-
- Swanson JM. Transdermal methylphenidate more effective than placebo for treating ADHD. Evidence Based Mental Health 2008;11(4):118. [PMID: ] - PubMed
van der Feltz‐Cornelis 2006 {published data only}
-
- Feltz-Cornelis C M, Aldenkamp A P. Effectiveness and safety of methylphenidate in adult attention deficit hyperactivity disorder in patients with epilepsy: an open treatment trial [Erratum appears in Epilepsy & Behavior, 2006 Nov;9(3):542]. Epilepsy & Behavior 2006;8(3):659-62. [PMID: ] - PubMed
Villanueva 2016 {published data only}
-
- Villanueva V, Garces M, Lopez-Gonzalez FJ, Rodriguez-Osorio X, Toledo M, Salas-Puig J, et al. Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: the FYDATA study. Epilepsy Research 2016;126:201-10. [PMID: ] - PubMed
Wernicke 2007 {published data only}
-
- Wernicke JF, Holdridge KC, Jin L, Edison T, Zhang S, Bangs ME, et al. Seizure risk in patients with attention-deficit-hyperactivity disorder treated with atomoxetine. Developmental Medicine & Child Neurology 2007;49(7):498-502. [PMID: ] - PubMed
References to ongoing studies
NCT02348073 2015 {published data only}
-
- NCT02348073. Efficacy of phosphatidylserine enriched with n-3 PUFA supplementation on ADHD in children with epilepsy. clinicaltrials.gov/ct2/show/NCT02348073 (first received 28 January 2015).
Additional references
Achenbach 1991
-
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: Department of Psychiatry, University of Vermont, 1991. [ISBN: 9780938565086]
Barkley 1990
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86(2):184-92. [PMID: ] - PubMed
BNF 2017
-
- Joint Formulary Committee. BNF 73 (British National Formulary). London: Pharmaceutical Press, 2017. [ISBN: 978-0857112767]
Brikell 2018
Brown 2013
-
- Brown MG, Becker DA, Pollard JR, Anderson CT. The diagnosis and treatment of attention deficit hyperactivity disorder in patients with epilepsy. Current Neurology and Neuroscience Reports 2013;13(6):351. [PMID: ] - PubMed
Cardamone 2013
CDC 2017
-
- Centers for Disease Prevention and Control. Data and Statistics About ADHD. www.cdc.gov/ncbddd/adhd/data.html 2017.
Coghill 2014
-
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biological Psychiatry 2014;76(8):603-15. [DOI: 10.1016/j.biopsych.2013.10.005] [PMID: ] - DOI - PubMed
Cohen 2013
-
- Cohen R, Senecky Y, Shuper A, Inbar D, Chodick G, Shalev V, et al. Prevalence of epilepsy and attention-deficit hyperactivity disorder (ADHD): a population-based study. Journal of Child Neurology 2013;28(1):120-3. [PMID: ] - PubMed
Conners 2008
-
- Conners CK. Conners 3rd Edition. Toronto: Multi-Health Systems Inc, 2008.
Covidence [Computer program]
-
- Covidence. Version accessed 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Cramer 1998
-
- Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross‐cultural translations of a 31‐item quality of life in epilepsy inventory. Epilepsia 1998;39(1):81-8. [PMID: ] - PubMed
Czamara 2013
-
- Czamara D, Tiesler CM, Kohlböck G, Berdel D, Hoffmann B, Bauer CP, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One 2013;8(5):e63859. [DOI: 10.1371/journal.pone.0063859] [PMID: ] - DOI - PMC - PubMed
De Sousa 2012
DSM‐IV
-
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association, 1994. [ISBN: 0-89042-061-0]
DSM‐IV‐TR
-
- American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders Text Revision. 4th edition. Washington, DC: American Psychiatric Association, 2000. [ISBN: 978-0890420256]
DSM‐V
-
- American Psychiatric Association. DSM-V: Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Association, 2013. [ISBN: 978-0890425558]
DuPaul 1998
-
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York (NY): Guilford Press, 1998. [ISBN: 978-1572304239]
Epilepsy Foundation 2013
-
- Schachter SC, Shafer P, Sirven J. Who gets epilepsy? Available at www.epilepsy.com/learn/epilepsy-101/who-gets-epilepsy 2013.
EPOC 2017
-
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors: data collection form. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources... 2017.
FDA 2006
-
- US Food and Drug Administration, Eli Lilly and Company. Strattera (atomoxetine HCl). Available at www.accessdata.fda.gov/drugsatfda_docs/label/2007/021411s004s012s013s015... 2006.
Fisher 2014a
-
- Fisher R. Epilepsy: a new definition; 2014. Available at: www.epilepsy.com/article/2014/4/revised-definition-epilepsy.
Fisher 2014b
-
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55(4):475-82. [PMID: ] - PubMed
Fisher 2017
-
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58(4):522-30. [PMID: ] - PubMed
French 2011
Gau 2010
Glanville 2019
Gnanavel 2019
Goulardins 2015
-
- Goulardins JB, Rigoli D, Licari M, Piek JP, Hasue RH, Oosterlaan J, et al. Attention deficit hyperactivity disorder and developmental coordination disorder: two separate disorders or do they share a common etiology. Behavioural Brain Research 2015;292:484-92. [DOI: 10.1016/j.bbr.2015.07.009] [PMID: ] - DOI - PubMed
GRADEPro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gucuyener 2003
-
- Gucuyener K, Erdemoglu AK, Senol S, Serdaroglu A, Soysal S, Kockar AI. Use of methylphenidate for attention-deficit hyperactivity disorder in patients with epilepsy or electroencephalographic abnormalities. Journal of Child Neurology 2003;18(2):109-12. [PMID: ] - PubMed
Guy 1976
-
- Guy W. Clinical global impressions. In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976:217-222. [DHEW PUBLICATION NO. (ADM): 76-338] [URL: archive.org/details/ecdeuassessmentm1933guyw]
Hesdorffer 2004
-
- Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Archives of General Psychiatry 2004;61(7):731-6. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hosenbocus 2012
ICD‐10 1992
-
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [ISBN: 9241544228] [URL: apps.who.int/iris/handle/10665/37958]
ILAE 1989
-
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389-99. [PMID: ] - PubMed
Kattimani 2011
Kaufmann 2009
-
- Kaufmann R, Goldberg-Stern H, Shuper A. Attention-deficit disorders and epilepsy in childhood: incidence, causative relations and treatment possibilities. Journal of Child Neurology 2009;24(6):727-33. [PMID: ] - PubMed
Landgraf 1998
-
- Landgraf JM, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Quality of Life Research 1998;7(5):433-45. [PMID: ] - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Leisman 2014
Leitner 2014
Lundh 2017
Marcus 2012
Markowitz 2001
-
- Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clinical Pharmacokinetics 2001;40(10):753-72. [PMID: ] - PubMed
Martel 2012
Mlinar 2016
Murray 2012
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197-223. [PMID: ] - PubMed
National Alliance on Mental Illness 2017
-
- National Alliance on Mental Illness. Attention deficit hyperactivity disorder (ADHD). Available at: www.nami.org/Learn-More/Mental-Health-Conditions/ADHD; 2017.
Ngugi 2010
NHS 2016
-
- NHS Choices. Attention deficit hyperactivity disorder (ADHD) — causes. www.nhs.uk/Conditions/Attention-deficit-hyperactivity-disorder/Pages/Cau... 2016.
NICE 2008
-
- National Institue for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. www.nice.org.uk/guidance/ng87 2008. [NICE GUIDELINE: NG87] - PubMed
NIMH 2016
-
- National Institute of Mental Health. Attention-Deficit/Hyperactivity Disorder; 2016. Available at: www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-....
Ottman 2005
Picot 2008
-
- Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 2008;49(7):1230-8. [PMID: ] - PubMed
Polanczyk 2007
Radziuk 2015
-
- Radziuk AL, Kieling RR, Santos K, Rotert R, Bastos F, Palmini AL. Methylphenidate improves the quality of life of children and adolescents with ADHD and difficult-to-treat epilepsies. Epilepsy & Behaviour 2015;46:215-20. [PMID: ] - PubMed
Rang 2011
-
- Rang HP, Dale MM, Ritter J, Flower RJ, Henderson G. CNS stimulants and psychotomimetic drugs. In: Rang and Dale's Pharmacology. 7th edition. Edinburgh; New York: Elsevier/Churchill Livingstone, 2011. [ISBN: 9780702034718]
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rhodes 2004
Rhodes 2005
Rhodes 2006
-
- Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug-naïve boys with ADHD II — broader executive and non-executive domains. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2006;47(11):1184-94. [DOI: 10.1111/j.1469-7610.2006.01633.x] [PMID: ] - DOI - PubMed
Rhodes 2012
-
- Rhodes SM, Park J, Seth S, Coghill DR. A comprehensive investigation of memory impairment in attention deficit hyperactivity disorder and oppositional defiant disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2012;53(2):128-37. [DOI: 10.1111/j.1469-7610.2011.02436.x] [PMID: ] - DOI - PubMed
Rosenau 2021
-
- Rosenau PT, Openneer TJ, Matthijssen AM, de Loo-Neus GH, Buitelaar JK, den Hoofdakker BJ, et al. Effects of methylphenidate on executive functioning in children and adolescents with ADHD after long-term use: a randomized, placebo-controlled discontinuation study. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2021;62(12):1444-52. [DOI: 10.1111/jcpp.13419] [PMID: ] - DOI - PMC - PubMed
Russ 2012
-
- Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012;129(2):256-64. [PMID: ] - PubMed
Russell 2014
Sander 2003
-
- Sander, JW. The epidemiology of epilepsy revisited. Current Opinion in Neurology 2003;16(2):165-70. [PMID: ] - PubMed
Sander 2009
-
- Sander JW, Rugg-Gunn FJ, Smalls JC, editor(s). Epilepsy 2009: From Benchside to Bedside - a Practical Guide to Epilepsy. 12th edition. National Society for Epilepsy, 2009. [ISBN: 978-0951955260]
Sauer 2005
-
- Sauer JM, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clinical Pharmacokinetics 2005;44(6):571-90. [PMID: ] - PubMed
Sayal 2018
Scheffer 2017
Schunemann 2019
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6/.
Sheslow 2003
-
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning, Second Edition (WRAML2); 2003. Available from: www.pearsonclinical.co.uk/Education/Assessments/memory-assessments/wraml....
Shier 2013
Smith 2010
-
- Smith ML. Neuropsychology in epilepsy: children are not small adults. Epilepsia 2010;51(Suppl 1):68-9. [PMID: ] - PubMed
Storebø 2015
Thapar 2013
Torres 2008
-
- Torres AR, Whitney J, Gonzalez-Heydrich J. Attention-deficit/hyperactivity disorder in pediatric patients with epilepsy: review of pharmacological treatment. Epilepsy & Behavior 2008;12(2):217-233. [PMID: ] - PubMed
Urion 2016
-
- Urion DK. Attention-Deficit/Hyperactivity Disorder. In: Kliegman R, Stanton B, Behrman RE, St Geme III JW, Schor NF, Nelson WE, editors(s). Nelson Textbook of Pediatrics. 20th edition. Vol. 1. Philadelphia (PA): Elsevier, 2016:200-4. [ISBN: 9781455775668]
Wechsler 1997
-
- Wechsler D. WAIS-III: administration and scoring manual: Wechsler Adult Intelligence Scale. 3rd edition. San Antonio (TX): The Psychological Corporation, 1997. [ISBN: 9780158981031]
Williams 2016
World Bank 2018
-
- World Bank. World Bank country and lending groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-coun... 2018.

